PET-CT in Clinical Adult Oncology-IV. Gynecologic and Genitourinary Malignancies
- PMID: 35740665
- PMCID: PMC9220973
- DOI: 10.3390/cancers14123000
PET-CT in Clinical Adult Oncology-IV. Gynecologic and Genitourinary Malignancies
Abstract
Concurrently acquired positron emission tomography and computed tomography (PET-CT) is an advanced imaging modality with diverse oncologic applications, including staging, therapeutic assessment, restaging and longitudinal surveillance. This series of six review articles focuses on providing practical information to providers and imaging professionals regarding the best use and interpretative strategies of PET-CT for oncologic indications in adult patients. In this fourth article of the series, the more common gynecological and adult genitourinary malignancies encountered in clinical practice are addressed, with an emphasis on Food and Drug Administration (FDA)-approved and clinically available radiopharmaceuticals. The advent of new FDA-approved radiopharmaceuticals for prostate cancer imaging has revolutionized PET-CT imaging in this important disease, and these are addressed in this report. However, [18F]F-fluoro-2-deoxy-d-glucose (FDG) remains the mainstay for PET-CT imaging of gynecologic and many other genitourinary malignancies. This information will serve as a guide for the appropriate role of PET-CT in the clinical management of gynecologic and genitourinary cancer patients for health care professionals caring for adult cancer patients. It also addresses the nuances and provides guidance in the accurate interpretation of FDG PET-CT in gynecological and genitourinary malignancies for imaging providers, including radiologists, nuclear medicine physicians and their trainees.
Keywords: PET; [18F]-fluorodeoxyglucose; fluciclovine; gynecologic malignancy; penile cancer; prostate cancer; prostate specific membrane antigen (PSMA); renal cell carcinoma; testicular cancer; urothelial carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

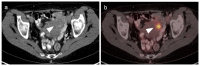



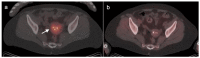
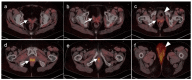
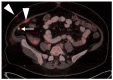



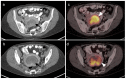

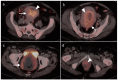
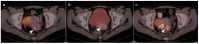

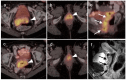




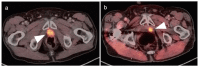


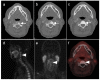

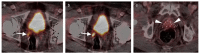

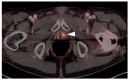
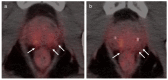

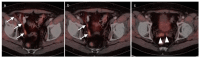
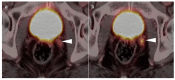
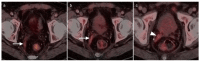





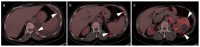
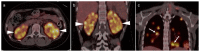
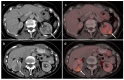
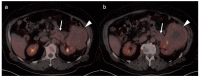




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

