Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States
- PMID: 35740866
- PMCID: PMC9221282
- DOI: 10.3390/biom12060740
Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States
Abstract
The mouse neuromuscular junction (NMJ) has long been used as a model synapse for the study of neurotransmission in both healthy and disease states of the NMJ. Neurotransmission from these neuromuscular nerve terminals occurs at highly organized structures called active zones (AZs). Within AZs, the relationships between the voltage-gated calcium channels and docked synaptic vesicles govern the probability of acetylcholine release during single action potentials, and the short-term plasticity characteristics during short, high frequency trains of action potentials. Understanding these relationships is important not only for healthy synapses, but also to better understand the pathophysiology of neuromuscular diseases. In particular, we are interested in Lambert-Eaton myasthenic syndrome (LEMS), an autoimmune disorder in which neurotransmitter release from the NMJ decreases, leading to severe muscle weakness. In LEMS, the reduced neurotransmission is traditionally thought to be caused by the antibody-mediated removal of presynaptic voltage-gated calcium channels. However, recent experimental data and AZ computer simulations have predicted that a disruption in the normally highly organized active zone structure, and perhaps autoantibodies to other presynaptic proteins, contribute significantly to pathological effects in the active zone and the characteristics of chemical transmitters.
Keywords: Lambert-Eaton myasthenic syndrome; active zone; computational modeling; neuromuscular junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

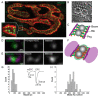
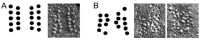
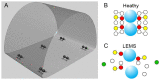
References
-
- Laghaei R., Ma J., Tarr T.B., Homan A.E., Kelly L.S., Tilvawala M.S., Vuocolo B.S., Rajasekaran H.P., Meriney S.D., Dittrich M. Transmitter release site organization can predict synaptic function at the neuromuscular junction. J. Neurophysiol. 2018;119:1340–1355. doi: 10.1152/jn.00168.2017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

