Gut Steroids and Microbiota: Effect of Gonadectomy and Sex
- PMID: 35740892
- PMCID: PMC9220917
- DOI: 10.3390/biom12060767
Gut Steroids and Microbiota: Effect of Gonadectomy and Sex
Abstract
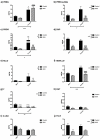
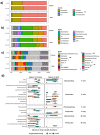
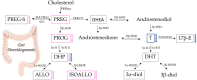
Sex steroids, derived mainly from gonads, can shape microbiota composition; however, the impact of gonadectomy and sex on steroid production in the gut (i.e., gut steroids), and its interaction with microbiota composition, needs to be clarified. In this study, steroid environment and gut steroidogenesis were analysed by liquid chromatography tandem mass spectrometry and expression analyses. Gut microbiota composition as branched- and short-chain fatty acids were determined by 16S rRNA gene sequence analysis and gas chromatography flame ionisation detection, respectively. Here, we first demonstrated that levels of pregnenolone (PREG), progesterone (PROG), and isoallopregnanolone (ISOALLO) were higher in the female rat colon, whereas the level of testosterone (T) was higher in males. Sexual dimorphism on gut steroidogenesis is also reported after gonadectomy. Sex, and more significantly, gonadectomy, affects microbiota composition. We noted that a number of taxa and inferred metabolic pathways were associated with gut steroids, such as positive associations between Blautia with T, dihydroprogesterone (DHP), and allopregnanolone (ALLO), whereas negative associations were noted between Roseburia and T, ALLO, PREG, ISOALLO, DHP, and PROG. In conclusion, this study highlights the novel sex-specific association between microbiota and gut steroids with possible relevance for the gut-brain axis.
Keywords: branched- and short-chain fatty acids; gastrointestinal tract; gut microbiota; mucosa; pregnenolone; sex dimorphism; sex steroids; stool.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Gut microbiota composition is altered in a preclinical model of type 1 diabetes mellitus: Influence on gut steroids, permeability, and cognitive abilities.Neuropharmacology. 2023 Mar 15;226:109405. doi: 10.1016/j.neuropharm.2022.109405. Epub 2022 Dec 23. Neuropharmacology. 2023. PMID: 36572179
-
3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period.Gynecol Endocrinol. 2005 Nov;21(5):268-79. doi: 10.1080/09513590500361747. Gynecol Endocrinol. 2005. PMID: 16373246
-
Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry.Endocrinology. 2006 Apr;147(4):1847-59. doi: 10.1210/en.2005-0955. Epub 2006 Jan 5. Endocrinology. 2006. PMID: 16396987
-
Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions.Prog Neurobiol. 2014 Feb;113:56-69. doi: 10.1016/j.pneurobio.2013.07.006. Epub 2013 Aug 16. Prog Neurobiol. 2014. PMID: 23958466 Review.
-
Biosynthesis and assay of neurosteroids in rats and mice: functional correlates.J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):355-60. doi: 10.1016/0960-0760(95)00074-a. J Steroid Biochem Mol Biol. 1995. PMID: 7626480 Review.
Cited by
-
Special series on the role of the microbiome in reproduction and fertility.Reprod Fertil. 2023 Nov 30;4(4):e230080. doi: 10.1530/RAF-23-0080. Print 2023 Oct 1. Reprod Fertil. 2023. PMID: 37947768 Free PMC article.
-
Gut Microbiota in Women with Eating Disorders: A New Frontier in Pathophysiology and Treatment.Nutrients. 2025 Jul 14;17(14):2316. doi: 10.3390/nu17142316. Nutrients. 2025. PMID: 40732941 Free PMC article. Review.
-
The Molecular Gut-Brain Axis in Early Brain Development.Int J Mol Sci. 2022 Dec 6;23(23):15389. doi: 10.3390/ijms232315389. Int J Mol Sci. 2022. PMID: 36499716 Free PMC article. Review.
-
Sex hormones, sex chromosomes, and microbiota: Identification of Akkermansia muciniphila as an estrogen-responsive microbiota.Microbiota Host. 2023;1(1):e230010. doi: 10.1530/mah-23-0010. Epub 2023 Oct 9. Microbiota Host. 2023. PMID: 37937163 Free PMC article.
-
Neuroactive Steroid-Gut Microbiota Interaction in T2DM Diabetic Encephalopathy.Biomolecules. 2023 Aug 29;13(9):1325. doi: 10.3390/biom13091325. Biomolecules. 2023. PMID: 37759725 Free PMC article.

