Filterability of Erythrocytes in Patients with COVID-19
- PMID: 35740907
- PMCID: PMC9220947
- DOI: 10.3390/biom12060782
Filterability of Erythrocytes in Patients with COVID-19
Abstract
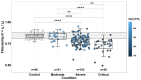
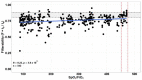
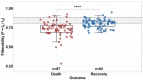
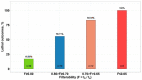
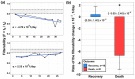
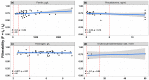
For the first time, the influence of COVID-19 on blood microrheology was studied. For this, the method of filtering erythrocytes through filters with pores of 3.5 μm was used. Filterability was shown to significantly decrease with the increasing severity of the patient's condition, as well as with a decrease in the ratio of hemoglobin oxygen saturation to the oxygen fraction in the inhaled air (SpO2/FiO2). The filterability of ≤ 0.65, or its fast decrease during treatment, were indicators of a poor prognosis. Filterability increased significantly with an increase in erythrocyte count, hematocrit and blood concentrations of hemoglobin, albumin, and total protein. The effect of these parameters on the erythrocyte filterability is directly opposite to their effect on blood macrorheology, where they all increase blood viscosity, worsening the erythrocyte deformability. The erythrocyte filterability decreased with increasing oxygen supply rate, especially in patients on mechanical ventilation, apparently not due to the oxygen supplied, but to the deterioration of the patients' condition. Filterability significantly correlates with the C-reactive protein, which indicates that inflammation affects the blood microrheology in the capillaries. Thus, the filterability of erythrocytes is a good tool for studying the severity of the patient's condition and his prognosis in COVID-19.
Keywords: COVID-19; SpO2/FiO2 ratio; additional oxygenation; dynamics of filterability alteration; erythrocyte; filterability; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- COVID-19 Coronavirus Pandemic. Worldometer. [(accessed on 22 April 2022)]. Available online: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1?%20.
-
- Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., Rech R., Colombo R., Antinori S., Corbellino M., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. - DOI - PMC - PubMed
-
- Della Rocca D.G., Magnocavallo M., Lavalle C., Romero J., Forleo G.B., Tarantino N., Chimenti C., Alviz I., Gamero M.T., Mario J., et al. Evidence of systemic endothelial injury and microthrombosis in hospitalized COVID-19 patients at different stages of the disease. J. Thromb. Thrombolysis. 2021;51:571–576. doi: 10.1007/s11239-020-02330-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

