Gamma-Linolenic Acid (GLA) Protects against Ionizing Radiation-Induced Damage: An In Vitro and In Vivo Study
- PMID: 35740923
- PMCID: PMC9221136
- DOI: 10.3390/biom12060797
Gamma-Linolenic Acid (GLA) Protects against Ionizing Radiation-Induced Damage: An In Vitro and In Vivo Study
Abstract
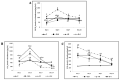
Radiation is pro-inflammatory in nature in view of its ability to induce the generation of reactive oxygen species (ROS), cytokines, chemokines, and growth factors with associated inflammatory cells. Cells are efficient in repairing radiation-induced DNA damage; however, exactly how this happens is not clear. In the present study, GLA reduced DNA damage (as evidenced by micronuclei formation) and enhanced metabolic viability, which led to an increase in the number of surviving RAW 264.7 cells in vitro by reducing ROS generation, and restoring the activities of desaturases, COX-1, COX-2, and 5-LOX enzymes, TNF-α/TGF-β, NF-kB/IkB, and Bcl-2/Bax ratios, and iNOS, AIM-2, and caspases 1 and 3, to near normal. These in vitro beneficial actions were confirmed by in vivo studies, which revealed that the survival of female C57BL/6J mice exposed to lethal radiation (survival~20%) is significantly enhanced (to ~80%) by GLA treatment by restoring altered levels of duodenal HMGB1, IL-6, TNF-α, and IL-10 concentrations, as well as the expression of NF-kB, IkB, Bcl-2, Bax, delta-6-desaturase, COX-2, and 5-LOX genes, and pro- and anti-oxidant enzymes (SOD, catalase, glutathione), to near normal. These in vitro and in vivo studies suggest that GLA protects cells/tissues from lethal doses of radiation by producing appropriate changes in inflammation and its resolution in a timely fashion.
Keywords: cytokines; inflammation; lipoxin A4; prostaglandins; radiation; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




















References
-
- Steel L.K., Hughes H.N., Walden T.L., Jr. Quantitative, functional and biochemical alterations in the peritoneal cells of mice exposed to whole-body gamma-irradiation. I. Changes in cellular protein, adherence properties and enzymatic activities associated with platelet-activating factor formation and inactivation, and arachidonate metabolism. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1988;53:943–964. - PubMed
-
- Bito L.Z., Klein E.M. The role of the arachidonic acid cascade in the species-specific X-ray-induced inflammation of the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1982;22:579–587. - PubMed
-
- Martinez R.M., Fattori V., Saito P., Melo C.B.P., Borghi S.M., Pinto I.C., Bussmann A.J.C., Baracat M.M., Georgetti S.R., Verri W.A., Jr., et al. Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice. J. Dermatol. Sci. 2018;S0923–S1811:30201–30209. doi: 10.1016/j.jdermsci.2018.04.014. - DOI - PubMed
-
- da-Palma-Cruz M., da Silva R.F., Monteiro D., Rehim H.M.M.A., Grabulosa C.C., de Oliveira A.P.L., Lino-Dos-Santos-Franco A. Photo biomodulation modulates the resolution of inflammation during acute lung injury induced by sepsis. Lasers Med. Sci. 2019;34:191–199. doi: 10.1007/s10103-018-2688-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

