Recent Insight into Lipid Binding and Lipid Modulation of Pentameric Ligand-Gated Ion Channels
- PMID: 35740939
- PMCID: PMC9221113
- DOI: 10.3390/biom12060814
Recent Insight into Lipid Binding and Lipid Modulation of Pentameric Ligand-Gated Ion Channels
Abstract
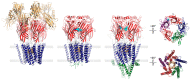
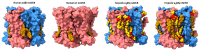
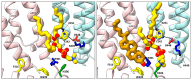
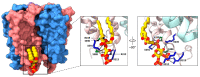
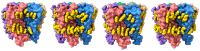
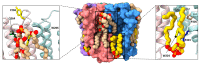
Pentameric ligand-gated ion channels (pLGICs) play a leading role in synaptic communication, are implicated in a variety of neurological processes, and are important targets for the treatment of neurological and neuromuscular disorders. Endogenous lipids and lipophilic compounds are potent modulators of pLGIC function and may help shape synaptic communication. Increasing structural and biophysical data reveal sites for lipid binding to pLGICs. Here, we update our evolving understanding of pLGIC-lipid interactions highlighting newly identified modes of lipid binding along with the mechanistic understanding derived from the new structural data.
Keywords: allosteric modulation; annular; lipid binding sites; lipid–protein interactions; non-annular; pentameric ligand-gated ion channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gibbs E., Chakrapani S. Structure, function and physiology of 5-hydroxytryptamine receptors subtype 3. In: Harris J.R., Marles-Wright J., editors. Macromolecular Protein Complexes III: Structure and Function. Subcellular Biochemistry. Springer International Publishing; Cham, Switzerland: 2021. pp. 373–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

