Occurrence of Lymphangiogenesis in Peripheral Nerve Autografts Contrasts Schwann Cell-Induced Apoptosis of Lymphatic Endothelial Cells In Vitro
- PMID: 35740945
- PMCID: PMC9221261
- DOI: 10.3390/biom12060820
Occurrence of Lymphangiogenesis in Peripheral Nerve Autografts Contrasts Schwann Cell-Induced Apoptosis of Lymphatic Endothelial Cells In Vitro
Abstract
Peripheral nerve injuries pose a major clinical concern world-wide, and functional recovery after segmental peripheral nerve injury is often unsatisfactory, even in cases of autografting. Although it is well established that angiogenesis plays a pivotal role during nerve regeneration, the influence of lymphangiogenesis is strongly under-investigated. In this study, we analyzed the presence of lymphatic vasculature in healthy and regenerated murine peripheral nerves, revealing that nerve autografts contained increased numbers of lymphatic vessels after segmental damage. This led us to elucidate the interaction between lymphatic endothelial cells (LECs) and Schwann cells (SCs) in vitro. We show that SC and LEC secretomes did not influence the respective other cell types' migration and proliferation in 2D scratch assay experiments. Furthermore, we successfully created lymphatic microvascular structures in SC-embedded 3D fibrin hydrogels, in the presence of supporting cells; whereas SCs seemed to exert anti-lymphangiogenic effects when cultured with LECs alone. Here, we describe, for the first time, increased lymphangiogenesis after peripheral nerve injury and repair. Furthermore, our findings indicate a potential lymph-repellent property of SCs, thereby providing a possible explanation for the lack of lymphatic vessels in the healthy endoneurium. Our results highlight the importance of elucidating the molecular mechanisms of SC-LEC interaction.
Keywords: Schwann cells; lymphangiogenesis; lymphatic endothelial cells; peripheral nerve regeneration.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

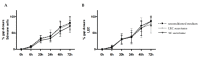

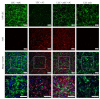



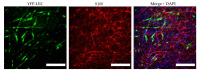
References
-
- López-Cebral R., Silva-Correia J.S., Reis R.L., Silva T.H., Oliveira J.M. Peripheral Nerve Injury: Current Challenges, Conventional Treatment Approaches, and New Trends in Biomaterials-Based Regenerative Strategies. ACS Biomater. Sci. Eng. 2017;3:3098–3122. doi: 10.1021/acsbiomaterials.7b00655. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

