NAFLD: Mechanisms, Treatments, and Biomarkers
- PMID: 35740949
- PMCID: PMC9221336
- DOI: 10.3390/biom12060824
NAFLD: Mechanisms, Treatments, and Biomarkers
Abstract
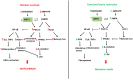
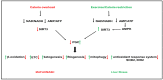
Nonalcoholic fatty liver disease (NAFLD), recently renamed metabolic-associated fatty liver disease (MAFLD), is one of the most common causes of liver diseases worldwide. NAFLD is growing in parallel with the obesity epidemic. No pharmacological treatment is available to treat NAFLD, specifically. The reason might be that NAFLD is a multi-factorial disease with an incomplete understanding of the mechanisms involved, an absence of accurate and inexpensive imaging tools, and lack of adequate non-invasive biomarkers. NAFLD consists of the accumulation of excess lipids in the liver, causing lipotoxicity that might progress to metabolic-associated steatohepatitis (NASH), liver fibrosis, and hepatocellular carcinoma. The mechanisms for the pathogenesis of NAFLD, current interventions in the management of the disease, and the role of sirtuins as potential targets for treatment are discussed here. In addition, the current diagnostic tools, and the role of non-coding RNAs as emerging diagnostic biomarkers are summarized. The availability of non-invasive biomarkers, and accurate and inexpensive non-invasive diagnosis tools are crucial in the detection of the early signs in the progression of NAFLD. This will expedite clinical trials and the validation of the emerging therapeutic treatments.
Keywords: biomarkers; lipotoxicity; liver; metabolic-associated fatty liver disease (MAFLD); metabolic-associated steatohepatitis (MASH); mitochondria; non-alcoholic fatty liver disease (NAFLD); non-alcoholic steatohepatitis (NASH); reactive oxygen species (ROS); sirtuins.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Eslam M., Sanyal A.J., George J., Sanyal A., Neuschwander-Tetri B., Tiribelli C., Kleiner D.E., Brunt E., Bugianesi E., Yki-Järvinen H., et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. - DOI - PubMed
-
- Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M., Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

