Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding
- PMID: 35740976
- PMCID: PMC9221196
- DOI: 10.3390/biom12060849
Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding
Abstract
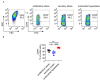
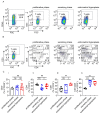
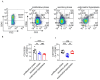
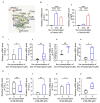
In patients, endometrial hyperplasia (EH) is often accompanied by abnormal uterine bleeding (AUB), which is prone to release large amounts of heme. However, the role of excess heme in the migration and infiltration of immune cells in EH complicated by AUB remains unknown. In this study, 45 patients with AUB were divided into three groups: a proliferative phase group (n = 15), a secretory phase group (n = 15) and EH (n = 15). We observed that immune cell subpopulations were significantly different among the three groups, as demonstrated by flow cytometry analysis. Of note, there was a higher infiltration of total immune cells and macrophages in the endometrium of patients with EH. Heme up-regulated the expression of heme oxygenase-1 (HO-1) and nuclear factor erythroid-2-related factor 2 (Nrf2) in endometrial epithelial cells (EECs) in vitro, as well as chemokine (e.g., CCL2, CCL3, CCL5, CXCL8) levels. Additionally, stimulation with heme led to the increased recruitment of THP-1 cells in an indirect EEC-THP-1 co-culture unit. These data suggest that sustained and excessive heme in patients with AUB may recruit macrophages by increasing the levels of several chemokines, contributing to the accumulation and infiltration of macrophages in the endometrium of EH patients, and the key molecules of heme metabolism, HO-1 and Nrf2, are also involved in this regulatory process.
Keywords: HO-1; abnormal uterine bleeding; endometrial hyperplasia; heme; immune cells; macrophages.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures



References
-
- Ottnad E., Parthasarathy S., Sambrano G.R., Ramprasad M.P., Quehenberger O., Kondratenko N., Green S., Steinberg D. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low density lipoprotein: Partial purification and role in recognition of oxidatively damaged cells. Proc. Natl. Acad. Sci. USA. 1995;92:1391–1395. doi: 10.1073/pnas.92.5.1391. - DOI - PMC - PubMed
-
- Acmaz G., Aksoy H., Unal D., Ozyurt S., Cingillioglu B., Aksoy U., Muderris I. Are neutrophil/lymphocyte and platelet/lymphocyte ratios associated with endometrial precancerous and cancerous lesions in patients with abnormal uterine bleeding? Asian Pac. J. Cancer Prev. 2014;15:1689–1692. doi: 10.7314/APJCP.2014.15.4.1689. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

