Critical Requirements for the Initiation of a Cardiac Arrhythmia in Rat Ventricle: How Many Myocytes?
- PMID: 35741007
- PMCID: PMC9221049
- DOI: 10.3390/cells11121878
Critical Requirements for the Initiation of a Cardiac Arrhythmia in Rat Ventricle: How Many Myocytes?
Abstract
Cardiovascular disease is the leading cause of death worldwide due in a large part to arrhythmia. In order to understand how calcium dynamics play a role in arrhythmogenesis, normal and dysfunctional Ca2+ signaling in a subcellular, cellular, and tissued level is examined using cardiac ventricular myocytes at a high temporal and spatial resolution using multiscale computational modeling. Ca2+ sparks underlie normal excitation-contraction coupling. However, under pathological conditions, Ca2+ sparks can combine to form Ca2+ waves. These propagating elevations of (Ca2+)i can activate an inward Na+-Ca2+ exchanger current (INCX) that contributes to early after-depolarization (EADs) and delayed after-depolarizations (DADs). However, how cellular currents lead to full depolarization of the myocardium and how they initiate extra systoles is still not fully understood. This study explores how many myocytes must be entrained to initiate arrhythmogenic depolarizations in biophysically detailed computational models. The model presented here suggests that only a small number of myocytes must activate in order to trigger an arrhythmogenic propagating action potential. These conditions were examined in 1-D, 2-D, and 3-D considering heart geometry. The depolarization of only a few hundred ventricular myocytes is required to trigger an ectopic depolarization. The number decreases under disease conditions such as heart failure. Furthermore, in geometrically restricted parts of the heart such as the thin muscle strands found in the trabeculae and papillary muscle, the number of cells needed to trigger a propagating depolarization falls even further to less than ten myocytes.
Keywords: arrhythmia; computational model; heart failure; ventricular myocyte network.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

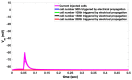
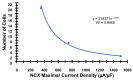



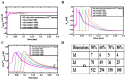

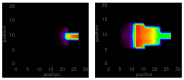


Similar articles
-
Calcium-Dependent Arrhythmogenic Foci Created by Weakly Coupled Myocytes in the Failing Heart.Circ Res. 2017 Dec 8;121(12):1379-1391. doi: 10.1161/CIRCRESAHA.117.312050. Epub 2017 Oct 2. Circ Res. 2017. PMID: 28970285 Free PMC article.
-
Na+ microdomains and sparks: Role in cardiac excitation-contraction coupling and arrhythmias in ankyrin-B deficiency.J Mol Cell Cardiol. 2019 Mar;128:145-157. doi: 10.1016/j.yjmcc.2019.02.001. Epub 2019 Feb 5. J Mol Cell Cardiol. 2019. PMID: 30731085
-
Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart.Circ Res. 2008 Aug 29;103(5):509-18. doi: 10.1161/CIRCRESAHA.108.176677. Epub 2008 Jul 17. Circ Res. 2008. PMID: 18635824
-
Do t-tubules play a role in arrhythmogenesis in cardiac ventricular myocytes?J Physiol. 2013 Sep 1;591(17):4141-7. doi: 10.1113/jphysiol.2013.254540. Epub 2013 May 7. J Physiol. 2013. PMID: 23652596 Free PMC article. Review.
-
Cardiac sodium transport and excitation-contraction coupling.J Mol Cell Cardiol. 2013 Aug;61:11-9. doi: 10.1016/j.yjmcc.2013.06.003. Epub 2013 Jun 14. J Mol Cell Cardiol. 2013. PMID: 23774049 Review.
Cited by
-
Local Control Model of a Human Ventricular Myocyte: An Exploration of Frequency-Dependent Changes and Calcium Sparks.Biomolecules. 2023 Aug 17;13(8):1259. doi: 10.3390/biom13081259. Biomolecules. 2023. PMID: 37627324 Free PMC article.
-
Pacing Dynamics Determines the Arrhythmogenic Mechanism of the CPVT2-Causing CASQ2G112+5X Mutation in a Guinea Pig Ventricular Myocyte Computational Model.Genes (Basel). 2022 Dec 22;14(1):23. doi: 10.3390/genes14010023. Genes (Basel). 2022. PMID: 36672764 Free PMC article.
-
Pathophysiology, molecular mechanisms, and genetics of atrial fibrillation.Hum Cell. 2024 Nov 6;38(1):14. doi: 10.1007/s13577-024-01145-z. Hum Cell. 2024. PMID: 39505800 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

