Immunogenicity of Novel AAV Capsids for Retinal Gene Therapy
- PMID: 35741009
- PMCID: PMC9221425
- DOI: 10.3390/cells11121881
Immunogenicity of Novel AAV Capsids for Retinal Gene Therapy
Abstract
Objectives: AAV vectors are widely used in gene therapy, but the prevalence of neutralizing antibodies raised against AAV serotypes in the course of a natural infection, as well as innate and adaptive immune responses induced upon vector administration, is still considered an important limitation. In ocular gene therapy, vectors applied subretinally bear the risk of retinal detachment or vascular leakage. Therefore, new AAV vectors that are suitable for intravitreal administration for photoreceptor transduction were developed.
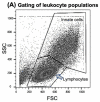
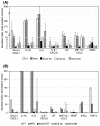
Methods: Here, we compared human immune responses from donors with suspected previous AAV2 infections to the new vectors AAV2.GL and AAV2.NN-two capsid peptide display variants with an enhanced tropism for photoreceptors-with the parental serotype AAV2 (AAV2 WT). We investigated total and neutralizing antibodies, adaptive and innate cellular immunogenicity determined by immunofluorescence staining and flow cytometry, and cytokine secretion analyzed with multiplex beads.
Results: While we did not observe obvious differences in overall antibody binding, variants-particularly AAV2.GL-were less sensitive to neutralizing antibodies than the AAV2 WT. The novel variants did not differ from AAV2 WT in cellular immune responses and cytokine production in vitro.
Conclusion: Due to their enhanced retinal tropism, which allows for dose reduction, the new vector variants are likely to be less immunogenic for gene therapy than the parental AAV2 vector.
Keywords: adaptive immunity; antibodies; cellular immunity; chemokines; cytokines; immunofluorescence; innate immunity; monocyte-derived DC; neutralization.
Conflict of interest statement
H.B. and S.M. are named inventors on the related patent application WO/2019/076856 covering the novel AAV capsids. S.M. is the co-founder of the gene therapy company ViGeneron GmbH, which owns the rights on the patent application WO/2019/076856. All other authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

