IGF2: Development, Genetic and Epigenetic Abnormalities
- PMID: 35741015
- PMCID: PMC9221339
- DOI: 10.3390/cells11121886
IGF2: Development, Genetic and Epigenetic Abnormalities
Abstract
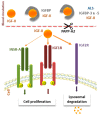
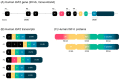
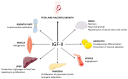
In the 30 years since the first report of parental imprinting in insulin-like growth factor 2 (Igf2) knockout mouse models, we have learnt much about the structure of this protein, its role and regulation. Indeed, many animal and human studies involving innovative techniques have shed light on the complex regulation of IGF2 expression. The physiological roles of IGF-II have also been documented, revealing pleiotropic tissue-specific and developmental-stage-dependent action. Furthermore, in recent years, animal studies have highlighted important interspecies differences in IGF-II function, gene expression and regulation. The identification of human disorders due to impaired IGF2 gene expression has also helped to elucidate the major role of IGF-II in growth and in tumor proliferation. The Silver-Russell and Beckwith-Wiedemann syndromes are the most representative imprinted disorders, as they constitute both phenotypic and molecular mirrors of IGF2-linked abnormalities. The characterization of patients with either epigenetic or genetic defects altering IGF2 expression has confirmed the central role of IGF-II in human growth regulation, particularly before birth, and its effects on broader body functions, such as metabolism or tumor susceptibility. Given the long-term health impact of these rare disorders, it is important to understand the consequences of IGF2 defects in these patients.
Keywords: Beckwith–Wiedemann syndrome; IGF2; Silver–Russell syndrome; growth; parental imprinting.
Conflict of interest statement
There is no conflict of interest to disclose related with this work for any of the authors.
Figures


References
-
- Giabicani E., Chantot-Bastaraud S., Bonnard A., Rachid M., Whalen S., Netchine I., Brioude F. Roles of Type 1 Insulin-Like Growth Factor (IGF) Receptor and IGF-II in Growth Regulation: Evidence from a Patient Carrying Both an 11p Paternal Duplication and 15q Deletion. Front. Endocrinol. 2019;10:263. doi: 10.3389/fendo.2019.00263. - DOI - PMC - PubMed
-
- Yu H., Berkel H. Insulin-like Growth Factors and Cancer. J. State Med. Soc. 1999;151:218–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

