Lethal and Non-Lethal Functions of Caspases in the DNA Damage Response
- PMID: 35741016
- PMCID: PMC9221191
- DOI: 10.3390/cells11121887
Lethal and Non-Lethal Functions of Caspases in the DNA Damage Response
Abstract
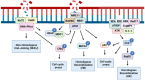
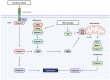
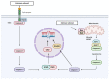
Members of the caspase family are well known for their roles in the initiation and execution of cell death. Due to their function in the removal of damaged cells that could otherwise become malignant, caspases are important players in the DNA damage response (DDR), a network of pathways that prevent genomic instability. However, emerging evidence of caspases positively or negatively impacting the accumulation of DNA damage in the absence of cell death demonstrates that caspases play a role in the DDR that is independent of their role in apoptosis. This review highlights the apoptotic and non-apoptotic roles of caspases in the DDR and how they can impact genomic stability and cancer treatment.
Keywords: DNA damage; apoptosis; caspases; cell cycle; genomic instability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

