Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells
- PMID: 35741021
- PMCID: PMC9221246
- DOI: 10.3390/cells11121892
Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells
Abstract
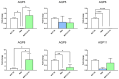
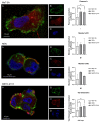
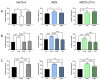
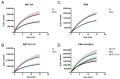
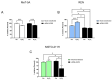
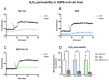
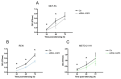
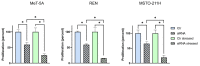
Malignant pleural mesothelioma (MPM) is an aggressive cancer of the pleural surface and is associated with previous asbestos exposure. The chemotherapy drug is one of the main treatments, but the median survival ranges from 8 to 14 months from diagnosis. The redox homeostasis of tumor cells should be carefully considered since elevated levels of ROS favor cancer cell progression (proliferation and migration), while a further elevation leads to ferroptosis. This study aims to analyze the functioning/role of aquaporins (AQPs) as a hydrogen peroxide (H2O2) channel in epithelial and biphasic MPM cell lines, as well as their possible involvement in chemotherapy drug resistance. Results show that AQP-3, -5, -6, -9, and -11 were expressed at mRNA and protein levels. AQP-6 was localized in the plasma membrane and intracellular structures. Compared to normal mesothelial cells, the water permeability of mesothelioma cells is not reduced by exogenous oxidative stress, but it is considerably increased by heat stress, making these cells resistant to ferroptosis. Functional experiments performed in mesothelioma cells silenced for aquaporin-6 revealed that it is responsible, at least in part, for the increase in H2O2 efflux caused by heat stress. Moreover, mesothelioma cells knocked down for AQP-6 showed a reduced proliferation compared to mock cells. Current findings suggest the major role of AQP-6 in providing mesothelioma cells with the ability to resist oxidative stress that underlies their resistance to chemotherapy drugs.
Keywords: Hyper7 probe; biphasic; epithelioid; gene silencing; hydrogen peroxide; peroxiporins; tumor proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cerium Oxide Nanoparticles Regulate Oxidative Stress in HeLa Cells by Increasing the Aquaporin-Mediated Hydrogen Peroxide Permeability.Int J Mol Sci. 2022 Sep 16;23(18):10837. doi: 10.3390/ijms231810837. Int J Mol Sci. 2022. PMID: 36142747 Free PMC article.
-
Aquaporin-1 expression in fluoro-edenite-induced mesothelioma effusions: An approach by cell-block procedure.Cytopathology. 2018 Oct;29(5):455-460. doi: 10.1111/cyt.12583. Epub 2018 Jul 23. Cytopathology. 2018. PMID: 29873855
-
Sigma-1 Receptor Agonists Acting on Aquaporin-Mediated H2O2 Permeability: New Tools for Counteracting Oxidative Stress.Int J Mol Sci. 2021 Sep 10;22(18):9790. doi: 10.3390/ijms22189790. Int J Mol Sci. 2021. PMID: 34575952 Free PMC article.
-
The established and future biomarkers of malignant pleural mesothelioma.Cancer Treat Rev. 2015 Jun;41(6):486-95. doi: 10.1016/j.ctrv.2015.05.001. Epub 2015 May 8. Cancer Treat Rev. 2015. PMID: 25979846 Review.
-
[Systemic Treatment of Malignant Pleural Mesothelioma].Gan To Kagaku Ryoho. 2017 Dec;44(13):2041-2047. Gan To Kagaku Ryoho. 2017. PMID: 29361614 Review. Japanese.
Cited by
-
Aquaglyceroporins in Human Breast Cancer.Cells. 2023 Aug 31;12(17):2185. doi: 10.3390/cells12172185. Cells. 2023. PMID: 37681917 Free PMC article.
-
Immunohistochemical Expression of Glutathione Peroxidase-2 (Gpx-2) and Its Clinical Relevance in Colon Adenocarcinoma Patients.Int J Mol Sci. 2023 Sep 27;24(19):14650. doi: 10.3390/ijms241914650. Int J Mol Sci. 2023. PMID: 37834097 Free PMC article.
-
Aquaporin water channels: roles beyond renal water handling.Nat Rev Nephrol. 2023 Sep;19(9):604-618. doi: 10.1038/s41581-023-00734-9. Epub 2023 Jul 17. Nat Rev Nephrol. 2023. PMID: 37460759 Review.
-
Critical Role of Aquaporins in Cancer: Focus on Hematological Malignancies.Cancers (Basel). 2022 Aug 29;14(17):4182. doi: 10.3390/cancers14174182. Cancers (Basel). 2022. PMID: 36077720 Free PMC article. Review.
-
Unexpected Classes of Aquaporin Channels Detected by Transcriptomic Analysis in Human Brain Are Associated with Both Patient Age and Alzheimer's Disease Status.Biomedicines. 2023 Mar 3;11(3):770. doi: 10.3390/biomedicines11030770. Biomedicines. 2023. PMID: 36979749 Free PMC article.
References
-
- PDQ Cancer Information Summaries Malignant Mesothelioma Treatment (Adult) (PDQ®)–Health Professional Version. PDQ Adult Treatment Editorial Board. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002–2022. Bookshelf ID: NBK65983. [(accessed on 28 April 2022)]; Available online: https://www.cancer.gov/types/mesothelioma/hp/mesothelioma-treatment-pdq.
-
- Mikulski S.M., Costanzi J.J., Vogelzang N.J., McCachren S., Taub R.N., Chun H., Mittelman A., Panella T., Puccio C., Fine R., et al. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J. Clin. Oncol. 2002;20:274–281. doi: 10.1200/JCO.2002.20.1.274. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

