RIP3 Translocation into Mitochondria Promotes Mitofilin Degradation to Increase Inflammation and Kidney Injury after Renal Ischemia-Reperfusion
- PMID: 35741025
- PMCID: PMC9220894
- DOI: 10.3390/cells11121894
RIP3 Translocation into Mitochondria Promotes Mitofilin Degradation to Increase Inflammation and Kidney Injury after Renal Ischemia-Reperfusion
Abstract
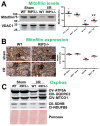
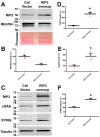
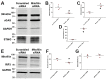
The receptor-interacting protein kinase 3 (RIP3) has been reported to regulate programmed necrosis-necroptosis forms of cell death with important functions in inflammation. We investigated whether RIP3 translocates into mitochondria in response to renal ischemia-reperfusion (I/R) to interact with inner mitochondrial protein (Mitofilin) and promote mtDNA release into the cytosol. We found that release of mtDNA activates the cGAS-STING pathway, leading to increased nuclear transcription of pro-inflammatory markers that exacerbate renal I/R injury. Monolateral C57/6N and RIP3-/- mice kidneys were subjected to 60 min of ischemia followed by either 12, 24, or 48 h of reperfusion. In WT mice, we found that renal I/R injury increased RIP3 levels, as well as its translocation into mitochondria. We observed that RIP3 interacts with Mitofilin, likely promoting its degradation, resulting in increased mitochondria damage and mtDNA release, activation of the cGAS-STING-p65 pathway, and increased transcription of pro-inflammatory markers. All of these effects observed in WT mice were decreased in RIP3-/- mice. In HK-2, RIP3 overexpression or Mitofilin knockdown increased cell death by activating the cGAS-STING-p65 pathway. Together, this study point to an important role of the RIP3-Mitofilin axis in the initiation and development of renal I/R injury.
Keywords: acute kidney injury (AKI); cGAS–STING–p65 pathway; inflammation; inner mitochondrial membrane protein (immt; mitochondrial dysfunction; mitochondrial structural integrity and function; mitofilin); mtDNA release; receptor-interacting protein kinase 3 (RIP3).
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Inhibition of Sirt3 activates the cGAS-STING pathway to aggravate hepatocyte damage in hepatic ischemia-reperfusion injury mice.Int Immunopharmacol. 2024 Feb 15;128:111474. doi: 10.1016/j.intimp.2023.111474. Epub 2024 Jan 6. Int Immunopharmacol. 2024. PMID: 38185036
-
Mitofilin Heterozygote Mice Display an Increase in Myocardial Injury and Inflammation after Ischemia/Reperfusion.Antioxidants (Basel). 2023 Apr 13;12(4):921. doi: 10.3390/antiox12040921. Antioxidants (Basel). 2023. PMID: 37107296 Free PMC article.
-
Blockade of the mitochondrial DNA release ameliorates hepatic ischemia-reperfusion injury through avoiding the activation of cGAS-Sting pathway.J Transl Med. 2024 Aug 28;22(1):796. doi: 10.1186/s12967-024-05588-8. J Transl Med. 2024. PMID: 39198913 Free PMC article.
-
cGAS-STING signaling in ischemic diseases.Clin Chim Acta. 2022 Jun 1;531:177-182. doi: 10.1016/j.cca.2022.04.003. Epub 2022 Apr 8. Clin Chim Acta. 2022. PMID: 35398249 Review.
-
The role of the kinases RIP1 and RIP3 in TNF-induced necrosis.Sci Signal. 2010 Mar 30;3(115):re4. doi: 10.1126/scisignal.3115re4. Sci Signal. 2010. PMID: 20354226 Review.
Cited by
-
cGAS-STING pathway in ischemia-reperfusion injury: a potential target to improve transplantation outcomes.Front Immunol. 2023 Sep 21;14:1231057. doi: 10.3389/fimmu.2023.1231057. eCollection 2023. Front Immunol. 2023. PMID: 37809088 Free PMC article. Review.
-
Suppressing mitochondrial inner membrane protein (IMMT) inhibits the proliferation of breast cancer cells through mitochondrial remodeling and metabolic regulation.Sci Rep. 2024 Jun 4;14(1):12766. doi: 10.1038/s41598-024-63427-8. Sci Rep. 2024. PMID: 38834715 Free PMC article.
-
Supervised Learning and Multi-Omics Integration Reveals Clinical Significance of Inner Membrane Mitochondrial Protein (IMMT) in Prognostic Prediction, Tumor Immune Microenvironment and Precision Medicine for Kidney Renal Clear Cell Carcinoma.Int J Mol Sci. 2023 May 15;24(10):8807. doi: 10.3390/ijms24108807. Int J Mol Sci. 2023. PMID: 37240154 Free PMC article.
-
Novel insight into cGAS-STING pathway in ischemic stroke: from pre- to post-disease.Front Immunol. 2023 Oct 17;14:1275408. doi: 10.3389/fimmu.2023.1275408. eCollection 2023. Front Immunol. 2023. PMID: 37915571 Free PMC article. Review.
-
Cytosolic nucleic acid sensing as driver of critical illness: mechanisms and advances in therapy.Signal Transduct Target Ther. 2025 Mar 19;10(1):90. doi: 10.1038/s41392-025-02174-2. Signal Transduct Target Ther. 2025. PMID: 40102400 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

