Alpha-Keratin, Keratin-Associated Proteins and Transglutaminase 1 Are Present in the Ortho- and Parakeratinized Epithelium of the Avian Tongue
- PMID: 35741029
- PMCID: PMC9221158
- DOI: 10.3390/cells11121899
Alpha-Keratin, Keratin-Associated Proteins and Transglutaminase 1 Are Present in the Ortho- and Parakeratinized Epithelium of the Avian Tongue
Abstract
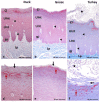
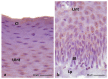
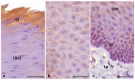
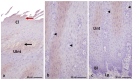
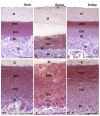
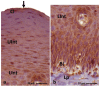
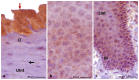
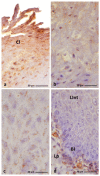
The lingual mucosa in birds is covered with two specific types of multilayered epithelia, i.e., the para- and orthokeratinized epithelium, that differ structurally and functionally. Comprehensive information on proteins synthesized in keratinocyte during their cytodifferentiation in subsequent layers of multilayered epithelia in birds concerns only the epidermis and are missing the epithelia of the lingual mucosa. The aim of the present study was to perform an immunohistochemical (IHC) and molecular analysis (WB) of bird-specific alpha-keratin, keratin-associated proteins (KAPs), namely filaggrin and loricrin, as well as transglutaminase 1 in the para- and orthokeratinized epithelium covering the tongue in the domestic duck, goose, and turkey. The results reveal the presence of alpha-keratin and KAPs in both epithelia, which is a sign of the cornification process. In contrast to the epidermis, the main KAPs involved in the cornification process of the lingual epithelia in birds is loricrin. Stronger expression with KAPs and transglutaminase 1 in the orthokeratinized epithelium than in the parakeratinized epithelium may determine the formation of a more efficient protective mechanical barrier. The presence of alpha-keratin, KAPs, and transglutaminase 1 epitopes characteristic of epidermal cornification in both types of the lingual epithelia may prove that they are of ectodermal origin.
Keywords: KAPs; alpha-keratin; birds; cornification; orthokeratinized epithelium; parakeratinized epithelium; tongue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










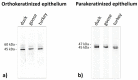
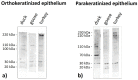
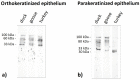

Similar articles
-
Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds.Cells. 2023 Jul 4;12(13):1776. doi: 10.3390/cells12131776. Cells. 2023. PMID: 37443811 Free PMC article.
-
Alpha-keratin and corneous beta protein in the parakeratinized epithelium of the tongue in the domestic goose (Anser anser f. domestica).J Exp Zool B Mol Dev Evol. 2019 Jul;332(5):158-166. doi: 10.1002/jez.b.22892. Epub 2019 Jun 26. J Exp Zool B Mol Dev Evol. 2019. PMID: 31243896
-
LM and TEM study of the orthokeratinized and parakeratinized epithelium of the tongue in the domestic duck (Anas platyrhynchos f. domestica).Micron. 2014 Dec;67:117-124. doi: 10.1016/j.micron.2014.07.004. Epub 2014 Jul 27. Micron. 2014. PMID: 25137178
-
Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia.J Anat. 2009 Apr;214(4):516-59. doi: 10.1111/j.1469-7580.2009.01066.x. J Anat. 2009. PMID: 19422428 Free PMC article. Review.
-
Vertebrate keratinization evolved into cornification mainly due to transglutaminase and sulfhydryl oxidase activities on epidermal proteins: An immunohistochemical survey.Anat Rec (Hoboken). 2022 Feb;305(2):333-358. doi: 10.1002/ar.24705. Epub 2021 Jul 29. Anat Rec (Hoboken). 2022. PMID: 34219408 Review.
Cited by
-
Transglutaminase Activity Is Conserved in Stratified Epithelia and Skin Appendages of Mammals and Birds.Int J Mol Sci. 2023 Jan 22;24(3):2193. doi: 10.3390/ijms24032193. Int J Mol Sci. 2023. PMID: 36768511 Free PMC article.
-
Pattern Distribution of Connexins in the Ortho- and Parakeratinized Epithelium of the Lingual Mucosa in Birds.Cells. 2023 Jul 4;12(13):1776. doi: 10.3390/cells12131776. Cells. 2023. PMID: 37443811 Free PMC article.
References
-
- Rhodin J.A.G., Reith E.J. Ultrastructure of keratin in oral mucosa, skin, esophagus, claw and hair. In: Butcher E.O., Signnaes R.F., editors. Fundamentals of Keratinization. American Association for the Advancement of Science; Washington, DC, USA: pp. 61–94.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

