The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction
- PMID: 35741047
- PMCID: PMC9222025
- DOI: 10.3390/cells11121919
The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction
Abstract
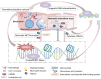
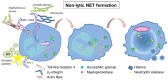
The innate immune system is the first line of defense against invading pathogens or sterile injuries. Pattern recognition receptors (PRR) sense molecules released from inflamed or damaged cells, or foreign molecules resulting from invading pathogens. PRRs can in turn induce inflammatory responses, comprising the generation of cytokines or chemokines, which further induce immune cell recruitment. Neutrophils represent an essential factor in the early immune response and fulfill numerous tasks to fight infection or heal injuries. The release of neutrophil extracellular traps (NETs) is part of it and was originally attributed to the capture and elimination of pathogens. In the last decade studies revealed a detrimental role of NETs during several diseases, often correlated with an exaggerated immune response. Overwhelming inflammation in single organs can induce remote organ damage, thereby further perpetuating release of inflammatory molecules. Here, we review recent findings regarding damage-associated molecular patterns (DAMPs) which are able to induce NET formation, as well as NET components known to act as DAMPs, generating a putative fatal circle of inflammation contributing to organ damage and sequentially occurring remote organ injury.
Keywords: CIRP; HMGB1; LL37; cfDNA; damage associated molecular pattern; histone; inflammation; innate immune response; neutrophil extracellular traps; remote organ damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

