Short-Chained Alcohols Make Membrane Surfaces Conducive for Melittin Action: Implication for the Physiological Role of Alcohols in Cells
- PMID: 35741057
- PMCID: PMC9221640
- DOI: 10.3390/cells11121928
Short-Chained Alcohols Make Membrane Surfaces Conducive for Melittin Action: Implication for the Physiological Role of Alcohols in Cells
Abstract
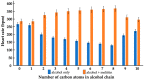
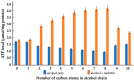
Alcohols are a part of cellular metabolism, but their physiological roles are not well understood. We investigated the effects of short-chain alcohols on Daphnia pulex and model membranes mimicking the lipid composition of eukaryotic inner mitochondrial membranes. We also studied the synergistic effects of alcohols with the bee venom membrane-active peptide, melittin, which is structurally similar to endogenous membrane-active peptides. The alcohols, from ethanol to octanol, gradually decreased the heart rate and the mitochondrial ATP synthesis of daphnia; in contrast, in combination with melittin, which exerted no sizeable effect, they gradually increased both the heart rate and the ATP synthesis. Lipid packing and the order parameter of oriented films, monitored by EPR spectroscopy of the spin-labeled probe 5-doxylstrearic acid, revealed gradual alcohol-assisted bilayer to non-bilayer transitions in the presence of melittin; further, while the alcohols decreased, in combination with melittin they increased the order parameter of the film, which is attributed to the alcohol-facilitated association of melittin with the membrane. A 1H-NMR spectroscopy of the liposomes confirmed the enhanced induction of a non-bilayer lipid phase that formed around the melittin, without the permeabilization of the liposomal membrane. Our data suggest that short-chain alcohols, in combination with endogenous peptides, regulate protein functions via modulating the lipid polymorphism of membranes.
Keywords: 1H-NMR; ERP of spin probes; alcohols; heart rate; melittin; mitochondrial ATP production; non-bilayer structures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Interaction of bee venom melittin with zwitterionic and negatively charged phospholipid bilayers: a spin-label electron spin resonance study.Biophys J. 1997 Feb;72(2 Pt 1):767-78. doi: 10.1016/s0006-3495(97)78711-3. Biophys J. 1997. PMID: 9017202 Free PMC article.
-
Bee Venom Melittin Disintegrates the Respiration of Mitochondria in Healthy Cells and Lymphoblasts, and Induces the Formation of Non-Bilayer Structures in Model Inner Mitochondrial Membranes.Int J Mol Sci. 2021 Oct 15;22(20):11122. doi: 10.3390/ijms222011122. Int J Mol Sci. 2021. PMID: 34681781 Free PMC article.
-
The actions of melittin on membranes.Biochim Biophys Acta. 1990 May 7;1031(2):143-61. doi: 10.1016/0304-4157(90)90006-x. Biochim Biophys Acta. 1990. PMID: 2187536 Review.
-
The structure of melittin in the form I crystals and its implication for melittin's lytic and surface activities.Biophys J. 1982 Jan;37(1):353-61. doi: 10.1016/S0006-3495(82)84683-3. Biophys J. 1982. PMID: 7055627 Free PMC article.
-
Bee Venom Components as Therapeutic Tools against Prostate Cancer.Toxins (Basel). 2021 May 7;13(5):337. doi: 10.3390/toxins13050337. Toxins (Basel). 2021. PMID: 34067049 Free PMC article. Review.
Cited by
-
In appreciation of an ingenious scientist and a great friend: Győző Garab.Photosynthetica. 2023 Oct 23;61(4):461-464. doi: 10.32615/ps.2023.040. eCollection 2023. Photosynthetica. 2023. PMID: 39649486 Free PMC article.
-
Cationic Proteins Rich in Lysine Residue Trigger Formation of Non-bilayer Lipid Phases in Model and Biological Membranes: Biophysical Methods of Study.J Membr Biol. 2023 Dec;256(4-6):373-391. doi: 10.1007/s00232-023-00292-y. Epub 2023 Sep 21. J Membr Biol. 2023. PMID: 37735238 Review.
References
-
- Yamamuro D., Yamazaki H., Osuga J., Okada K., Wakabayashi T., Takei A., Takei S., Takahashi M., Nagashima S., Holleboom A.G., et al. Esterification of 4β-hydroxycholesterol and other oxysterols in human plasma occurs independently of LCAT. J. Lipid Res. 2020;61:1287–1299. doi: 10.1194/jlr.RA119000512. - DOI - PMC - PubMed
-
- Hubel E., Fishman S., Holopainen M., Käkelä R., Shaffer O., Houri I., Zvibel I., Shibolet O. Repetitive amiodarone administration causes liver damage via adipose tissue ER stress-dependent lipolysis, leading to hepatotoxic free fatty acid accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2021;321:G298–G307. doi: 10.1152/ajpgi.00458.2020. - DOI - PubMed
-
- Federico A., Cotticelli G., Festi D., Schiumerini R., Addolorato G., Ferrulli A., Merli M., Lucidi C., Milani S., Panella C., et al. The effects of alcohol on gastrointestinal tract, liver and pancreas: Evidence-based suggestions for clinical management. Eur. Rev. Med. Pharm. Sci. 2015;19:1922–1940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

