Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation
- PMID: 35741064
- PMCID: PMC9221661
- DOI: 10.3390/cells11121935
Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation
Abstract
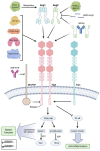
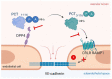
Systemic inflammation can be triggered by infection, surgery, trauma or burns. During systemic inflammation, an overshooting immune response induces tissue damage resulting in organ dysfunction and mortality. Endothelial cells make up the inner lining of all blood vessels and are critically involved in maintaining organ integrity by regulating tissue perfusion. Permeability of the endothelial monolayer is strictly controlled and highly organ-specific, forming continuous, fenestrated and discontinuous capillaries that orchestrate the extravasation of fluids, proteins and solutes to maintain organ homeostasis. In the physiological state, the endothelial barrier is maintained by the glycocalyx, extracellular matrix and intercellular junctions including adherens and tight junctions. As endothelial cells are constantly sensing and responding to the extracellular environment, their activation by inflammatory stimuli promotes a loss of endothelial barrier function, which has been identified as a hallmark of systemic inflammation, leading to tissue edema formation and hypotension and thus, is a key contributor to lethal outcomes. In this review, we provide a comprehensive summary of the major players, such as the angiopoietin-Tie2 signaling axis, adrenomedullin and vascular endothelial (VE-) cadherin, that substantially contribute to the regulation and dysregulation of endothelial permeability during systemic inflammation and elucidate treatment strategies targeting the preservation of vascular integrity.
Keywords: adrenomedullin; angiopoietin-Tie2; capillary leakage; endothelium; procalcitonin; systemic inflammation; vascular permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

