Generating iPSCs with a High-Efficient, Non-Invasive Method-An Improved Way to Cultivate Keratinocytes from Plucked Hair for Reprogramming
- PMID: 35741085
- PMCID: PMC9222083
- DOI: 10.3390/cells11121955
Generating iPSCs with a High-Efficient, Non-Invasive Method-An Improved Way to Cultivate Keratinocytes from Plucked Hair for Reprogramming
Abstract
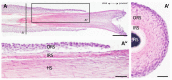
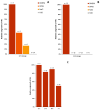
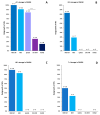
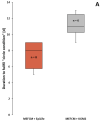
Various somatic cell types are suitable for induced pluripotency reprogramming, such as dermal fibroblasts, mesenchymal stem cells or hair keratinocytes. Harvesting primary epithelial keratinocytes from plucked human hair follicles (HFs) represents an easy and non-invasive alternative to a fibroblast culture from invasive skin biopsies. Nevertheless, to facilitate and simplify the process, which can be divided into three main steps (collecting, culturing and reprogramming), the whole procedure of generating hair keratinocytes has to be revised and upgraded continuously. In this study, we address advancements and approaches which improve the generation and handling of primary HF-derived keratinocytes tremendously, e.g., for iPSCs reprogramming. We not only evaluated different serum- and animal-origin-free media, but also supplements and coating solutions for an enhanced protocol. Here, we demonstrate the importance of speed and accuracy in the collecting step, as well as the choice of the right transportation medium. Our results lead to a more defined approach that further increases the reliability of downstream experiments and inter-laboratory reproducibility. These improvements will make it possible to obtain keratinocytes from plucked human hair for the generation of donor-specific iPSCs easier and more efficient than ever before, whilst preserving a non-invasive capability.
Keywords: hair follicle (HF); induced pluripotent stem cells (iPSCs); keratinocytes; plucked human hair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Sobhani A., Khanlarkhani N., Baazm M., Mohammadzadeh F., Najafi A., Mehdinejadiani S., Aval F.S. Multipotent Stem Cell and Current Application. Acta Med. Iran. 2017;55:6–23. - PubMed
-
- Shafa M., Yang F., Fellner T., Rao M.S., Baghbaderani B.A. Human-Induced Pluripotent Stem Cells Manufactured Using a Current Good Manufacturing Practice-Compliant Process Differentiate Into Clinically Relevant Cells From Three Germ Layers. Front. Med. 2018;5:69. doi: 10.3389/fmed.2018.00069. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

