Dysregulated Ca2+ Homeostasis as a Central Theme in Neurodegeneration: Lessons from Alzheimer's Disease and Wolfram Syndrome
- PMID: 35741091
- PMCID: PMC9221778
- DOI: 10.3390/cells11121963
Dysregulated Ca2+ Homeostasis as a Central Theme in Neurodegeneration: Lessons from Alzheimer's Disease and Wolfram Syndrome
Abstract
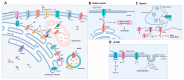
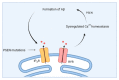
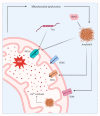
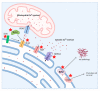
Calcium ions (Ca2+) operate as important messengers in the cell, indispensable for signaling the underlying numerous cellular processes in all of the cell types in the human body. In neurons, Ca2+ signaling is crucial for regulating synaptic transmission and for the processes of learning and memory formation. Hence, the dysregulation of intracellular Ca2+ homeostasis results in a broad range of disorders, including cancer and neurodegeneration. A major source for intracellular Ca2+ is the endoplasmic reticulum (ER), which has close contacts with other organelles, including mitochondria. In this review, we focus on the emerging role of Ca2+ signaling at the ER-mitochondrial interface in two different neurodegenerative diseases, namely Alzheimer's disease and Wolfram syndrome. Both of these diseases share some common hallmarks in the early stages, including alterations in the ER and mitochondrial Ca2+ handling, mitochondrial dysfunction and increased Reactive oxygen species (ROS) production. This indicates that similar mechanisms may underly these two disease pathologies and suggests that both research topics might benefit from complementary research.
Keywords: Alzheimer’s disease; Wolfram syndrome; calcium; mitochondria; mitochondria-associated ER membranes (MAMs); neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

