When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers
- PMID: 35741097
- PMCID: PMC9222101
- DOI: 10.3390/cells11121968
When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers
Abstract
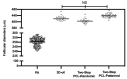
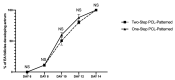
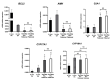
Current assisted reproduction technologies (ART) are insufficient to cover the slice of the population needing to restore fertility, as well as to amplify the reproductive performance of domestic animals or endangered species. The design of dedicated reproductive scaffolds has opened the possibility to better recapitulate the reproductive 3D ovarian environment, thus potentially innovating in vitro folliculogenesis (ivF) techniques. To this aim, the present research has been designed to compare ovine preantral follicles in vitro culture on poly(epsilon-caprolactone) (PCL)-based electrospun scaffolds designed with different topology (Random vs. Patterned fibers) with a previously validated system. The ivF performances were assessed after 14 days under 3D-oil, Two-Step (7 days in 3D-oil and on scaffold), or One-Step PCL protocols (14 days on PCL-scaffold) by assessing morphological and functional outcomes. The results show that Two- and One-Step PCL ivF protocols, when performed on patterned scaffolds, were both able to support follicle growth, antrum formation, and the upregulation of follicle marker genes leading to a greater oocyte meiotic competence than in the 3D-oil system. In conclusion, the One-Step approach could be proposed as a practical and valid strategy to support a synergic follicle-oocyte in vitro development, providing an innovative tool to enhance the availability of matured gametes on an individual basis for ART purposes.
Keywords: PCL-electrospun scaffold; artificial-ovary; in vitro follicle culture; oocyte meiotic competence; patterned fibers; preantral follicle growth; random fibers; sheep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Scaramuzzi R.J., Baird D.T., Campbell B.K., Driancourt M.A., Dupont J., Fortune J.E., Gilchrist R.B., Martin G.B., McNatty K.P., McNeilly A.S., et al. Regulation of Folliculogenesis and the Determination of Ovulation Rate in Ruminants. Reprod. Fertil. Dev. 2011;23:444–467. doi: 10.1071/RD09161. - DOI - PubMed
-
- Adamson G.D., de Mouzon J., Chambers G.M., Zegers-Hochschild F., Mansour R., Ishihara O., Banker M., Dyer S. International Committee for Monitoring Assisted Reproductive Technology: World Report on Assisted Reproductive Technology, 2011. Fertil. Steril. 2018;110:1067–1080. doi: 10.1016/j.fertnstert.2018.06.039. - DOI - PubMed
-
- Ori S. Global Trends in Reproductive Policy and Practice. 8th ed. Volume 4. IFFS; Royal, NJ, USA: 2019. International Federation of Fertility Societies’ Surveillance International Federation of Fertility Societies’ Surveillance (IFFS) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

