Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis
- PMID: 35741112
- PMCID: PMC9222155
- DOI: 10.3390/diagnostics12061302
Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis
Abstract
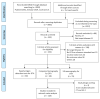
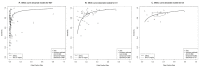
(1) Background: The comparative performance of various diagnostic methods for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection remains unclear. This study aimed to investigate the comparison of the 3 index test performances of rapid antigen diagnostic tests (RDTs), chest computed tomography (CT), and lung point-of-care-ultrasonography (US) with reverse transcription-polymerase chain reaction (RT-PCR), the reference standard, to provide more evidence-based data on the appropriate use of these index tests. (2) Methods: We retrieved data from electronic literature searches of PubMed, Cochrane Library, and EMBASE from 1 January 2020, to 1 April 2021. Diagnostic performance was examined using bivariate random-effects diagnostic test accuracy (DTA) and Bayesian network meta-analysis (NMA) models. (3) Results: Of the 3992 studies identified in our search, 118 including 69,445 participants met our selection criteria. Among these, 69 RDT, 38 CT, and 15 US studies in the pairwise meta-analysis were included for DTA with NMA. CT and US had high sensitivity of 0.852 (95% credible interval (CrI), 0.791-0.914) and 0.879 (95% CrI, 0.784-0.973), respectively. RDT had high specificity, 0.978 (95% CrI, 0.960-0.996). In accuracy assessment, RDT and CT had a relatively higher than US. However, there was no significant difference in accuracy between the 3 index tests. (4) Conclusions: This meta-analysis suggests that, compared with the reference standard RT-PCR, the 3 index tests (RDTs, chest CT, and lung US) had similar and complementary performances for diagnosis of SARS-CoV-2 infection. To manage and control COVID-19 effectively, future large-scale prospective studies could be used to obtain an optimal timely diagnostic process that identifies the condition of the patient accurately.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; computed tomography; imaging diagnostic test; meta-analysis; rapid antigen diagnostic test; systematic review; ultrasonography.
Conflict of interest statement
The authors of this manuscript declare no relationship with any interest related to the subject matter of the article. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD013639. doi: 10.1002/14651858.CD013639.pub4. PMID: 33242342 Updated.
-
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications.Microbiol Spectr. 2024 Jun 4;12(6):e0007324. doi: 10.1128/spectrum.00073-24. Epub 2024 Apr 29. Microbiol Spectr. 2024. PMID: 38683014 Free PMC article.
-
A Multicenter Clinical Diagnostic Accuracy Study of SureStatus, an Affordable, WHO Emergency Use-Listed, Rapid, Point-Of-Care Antigen-Detecting Diagnostic Test for SARS-CoV-2.Microbiol Spectr. 2022 Oct 26;10(5):e0122922. doi: 10.1128/spectrum.01229-22. Epub 2022 Sep 6. Microbiol Spectr. 2022. PMID: 36066256 Free PMC article.
-
Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: An updated systematic review and meta-analysis with meta-regression analyzing influencing factors.PLoS Med. 2022 May 26;19(5):e1004011. doi: 10.1371/journal.pmed.1004011. eCollection 2022 May. PLoS Med. 2022. PMID: 35617375 Free PMC article.
Cited by
-
Progress in Biosensors for the Point-of-Care Diagnosis of COVID-19.Sensors (Basel). 2022 Sep 29;22(19):7423. doi: 10.3390/s22197423. Sensors (Basel). 2022. PMID: 36236521 Free PMC article. Review.
-
Clinical impact of rapid molecular diagnostic tests in patients presenting with viral respiratory symptoms: A systematic literature review.PLoS One. 2024 Jun 13;19(6):e0303560. doi: 10.1371/journal.pone.0303560. eCollection 2024. PLoS One. 2024. PMID: 38870136 Free PMC article.
-
Diagnostic Accuracy of a SARS-CoV-2 Antigen Test Obtained by Mid-turbinate Nasal Swabs.Cureus. 2025 Jul 1;17(7):e87120. doi: 10.7759/cureus.87120. eCollection 2025 Jul. Cureus. 2025. PMID: 40747206 Free PMC article.
References
-
- World Health Organization Weekly Epidemiological Update on COVID-19. [(accessed on 6 January 2022)]. Available online: https://www.who.int/publications/m/item/weeklyoperational-update-on-covi....
-
- Lieberman J.A., Pepper G., Naccache S.N., Huang M.L., Jerome K.R., Greninger A.L. Comparison of Commercially Available and Laboratory-Developed Assays for In Vitro Detection of SARS-CoV-2 in Clinical Laboratories. J. Clin. Microbiol. 2020;58:e00821-20. doi: 10.1128/JCM.00821-20. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

