Low-Salt Diet Reduces Anti-CTLA4 Mediated Systemic Immune-Related Adverse Events while Retaining Therapeutic Efficacy against Breast Cancer
- PMID: 35741331
- PMCID: PMC9219826
- DOI: 10.3390/biology11060810
Low-Salt Diet Reduces Anti-CTLA4 Mediated Systemic Immune-Related Adverse Events while Retaining Therapeutic Efficacy against Breast Cancer
Abstract
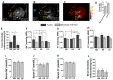
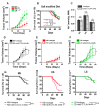
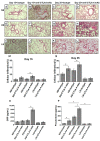
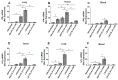
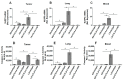
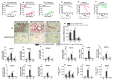
Immune checkpoint inhibitor (ICI) therapy has revolutionized the breast cancer treatment landscape. However, ICI-induced systemic inflammatory immune-related adverse events (irAE) remain a major clinical challenge. Previous studies in our laboratory and others have demonstrated that a high-salt (HS) diet induces inflammatory activation of CD4+T cells leading to anti-tumor responses. In our current communication, we analyzed the impact of dietary salt modification on therapeutic and systemic outcomes in breast-tumor-bearing mice following anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) monoclonal antibody (mAb) based ICI therapy. As HS diet and anti-CTLA4 mAb both exert pro-inflammatory activation of CD4+T cells, we hypothesized that a combination of these would lead to enhanced irAE response, while low-salt (LS) diet through blunting peripheral inflammatory action of CD4+T cells would reduce irAE response. We utilized an orthotopic murine breast tumor model by injecting Py230 murine breast cancer cells into syngeneic C57Bl/6 mice. In an LS diet cohort, anti-CTLA4 mAb treatment significantly reduced tumor progression (day 35, 339 ± 121 mm3), as compared to isotype mAb (639 ± 163 mm3, p < 0.05). In an HS diet cohort, treatment with anti-CTLA4 reduced the survival rate (day 80, 2/15) compared to respective normal/regular salt (NS) diet cohort (8/15, p < 0.05). Further, HS plus anti-CTLA4 mAb caused an increased expression of inflammatory cytokines (IFNγ and IL-1β) in lung infiltrating and peripheral circulating CD4+T cells. This inflammatory activation of CD4+T cells in the HS plus anti-CTLA4 cohort was associated with the upregulation of inflammasome complex activity. However, an LS diet did not induce any significant irAE response in breast-tumor-bearing mice upon treatment with anti-CTLA4 mAb, thus suggesting the role of high-salt diet in irAE response. Importantly, CD4-specific knock out of osmosensitive transcription factor NFAT5 using CD4cre/creNFAT5flox/flox transgenic mice caused a downregulation of high-salt-mediated inflammatory activation of CD4+T cells and irAE response. Taken together, our data suggest that LS diet inhibits the anti-CTLA4 mAb-induced irAE response while retaining its anti-tumor efficacy.
Keywords: breast cancer; cancer biology; cytokines; immunotherapy; t-helper cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Chronic High-Salt Diet Activates Tumor-Initiating Stem Cells Leading to Breast Cancer Proliferation.Cells. 2024 May 25;13(11):912. doi: 10.3390/cells13110912. Cells. 2024. PMID: 38891044 Free PMC article.
-
Ex Vivo High Salt Activated Tumor-Primed CD4+T Lymphocytes Exert a Potent Anti-Cancer Response.Cancers (Basel). 2021 Apr 2;13(7):1690. doi: 10.3390/cancers13071690. Cancers (Basel). 2021. PMID: 33918403 Free PMC article.
-
Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective.EClinicalMedicine. 2024 Mar 22;70:102536. doi: 10.1016/j.eclinm.2024.102536. eCollection 2024 Apr. EClinicalMedicine. 2024. PMID: 38560659 Free PMC article.
-
Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity.J Clin Invest. 2019 Jan 2;129(1):349-363. doi: 10.1172/JCI123391. Epub 2018 Dec 10. J Clin Invest. 2019. PMID: 30530991 Free PMC article.
-
Rheumatic immune-related adverse events associated with cancer immunotherapy: A nationwide multi-center cohort.Autoimmun Rev. 2020 Aug;19(8):102595. doi: 10.1016/j.autrev.2020.102595. Epub 2020 Jun 11. Autoimmun Rev. 2020. PMID: 32535092 Review.
Cited by
-
Stocking the toolbox-Using preclinical models to understand the development and treatment of immune checkpoint inhibitor-induced immune-related adverse events.Immunol Rev. 2023 Sep;318(1):110-137. doi: 10.1111/imr.13250. Epub 2023 Aug 10. Immunol Rev. 2023. PMID: 37565407 Free PMC article. Review.
-
Chronic High-Salt Diet Activates Tumor-Initiating Stem Cells Leading to Breast Cancer Proliferation.Cells. 2024 May 25;13(11):912. doi: 10.3390/cells13110912. Cells. 2024. PMID: 38891044 Free PMC article.
-
NFAT5: a stress-related transcription factor with multiple functions in health and disease.Cell Stress. 2025 May 22;9:16-48. doi: 10.15698/cst2025.05.304. eCollection 2025. Cell Stress. 2025. PMID: 40421201 Free PMC article. Review.
-
High-Salt Tumor Microenvironment: Not as Bad as It Sounds, Not as Good as It Seems.Cancers (Basel). 2025 Jun 10;17(12):1924. doi: 10.3390/cancers17121924. Cancers (Basel). 2025. PMID: 40563574 Free PMC article. Review.
-
Elucidation of clinical implications Arising from circadian rhythm and insights into the tumor immune landscape in breast cancer.Heliyon. 2024 Mar 6;10(6):e27356. doi: 10.1016/j.heliyon.2024.e27356. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38500978 Free PMC article.
References
-
- American Cancer Society . Cancer Facts & Figures (2022) American Cancer Society; Atlanta, GA, USA: 2022.
-
- Sivapiragasam A., Ashok Kumar P., Sokol E.S., Albacker L.A., Killian J.K., Ramkissoon S.H., Huang R.S.P., Severson E.A., Brown C.A., Danziger N., et al. Predictive Biomarkers for Im mune Checkpoint Inhibitors in Metastatic Breast Cancer. Cancer Med. 2021;10:53–61. doi: 10.1002/cam4.3550. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

