Binge-like Prenatal Ethanol Exposure Causes Impaired Cellular Differentiation in the Embryonic Forebrain and Synaptic and Behavioral Defects in Adult Mice
- PMID: 35741678
- PMCID: PMC9220802
- DOI: 10.3390/brainsci12060793
Binge-like Prenatal Ethanol Exposure Causes Impaired Cellular Differentiation in the Embryonic Forebrain and Synaptic and Behavioral Defects in Adult Mice
Abstract
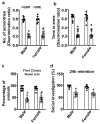
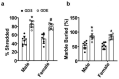
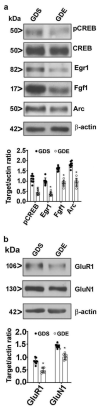
An embryo's in-utero exposure to ethanol due to a mother's alcohol drinking results in a range of deficits in the child that are collectively termed fetal alcohol spectrum disorders (FASDs). Prenatal ethanol exposure is one of the leading causes of preventable intellectual disability. Its neurobehavioral underpinnings warrant systematic research. We investigated the immediate effects on embryos of acute prenatal ethanol exposure during gestational days (GDs) and the influence of such exposure on persistent neurobehavioral deficits in adult offspring. We administered pregnant C57BL/6J mice with ethanol (1.75 g/kg) (GDE) or saline (GDS) intraperitoneally (i.p.) at 0 h and again at 2 h intervals on GD 8 and GD 12. Subsequently, we assessed apoptosis, differentiation, and signaling events in embryo forebrains (E13.5; GD13.5). Long-lasting effects of GDE were evaluated via a behavioral test battery. We also determined the long-term potentiation and synaptic plasticity-related protein expression in adult hippocampal tissue. GDE caused apoptosis, inhibited differentiation, and reduced pERK and pCREB signaling and the expression of transcription factors Pax6 and Lhx2. GDE caused persistent spatial and social investigation memory deficits compared with saline controls, regardless of sex. Interestingly, GDE adult mice exhibited enhanced repetitive and anxiety-like behavior, irrespective of sex. GDE reduced synaptic plasticity-related protein expression and caused hippocampal synaptic plasticity (LTP and LTD) deficits in adult offspring. These findings demonstrate that binge-like ethanol exposure at the GD8 and GD12 developmental stages causes defects in pERK-pCREB signaling and reduces the expression of Pax6 and Lhx2, leading to impaired cellular differentiation during the embryonic stage. In the adult stage, binge-like ethanol exposure caused persistent synaptic and behavioral abnormalities in adult mice. Furthermore, the findings suggest that combining ethanol exposure at two sensitive stages (GD8 and GD12) causes deficits in synaptic plasticity-associated proteins (Arc, Egr1, Fgf1, GluR1, and GluN1), leading to persistent FASD-like neurobehavioral deficits in mice.
Keywords: FASD; alcohol; brain; cognition; development; disabilities; electrophysiology; gestation; obsessive-compulsive disorder; psychiatric disorders; synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Activity-dependent Signaling and Epigenetic Abnormalities in Mice Exposed to Postnatal Ethanol.Neuroscience. 2018 Nov 10;392:230-240. doi: 10.1016/j.neuroscience.2018.07.011. Epub 2018 Jul 20. Neuroscience. 2018. PMID: 30031835 Free PMC article.
-
Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure.Neurosci Lett. 2013 Sep 13;551:7-11. doi: 10.1016/j.neulet.2013.05.051. Epub 2013 Jul 18. Neurosci Lett. 2013. PMID: 23872044
-
Postnatal Ethanol Exposure Activates HDAC-Mediated Histone Deacetylation, Impairs Synaptic Plasticity Gene Expression and Behavior in Mice.Int J Neuropsychopharmacol. 2020 May 27;23(5):324-338. doi: 10.1093/ijnp/pyaa017. Int J Neuropsychopharmacol. 2020. PMID: 32170298 Free PMC article.
-
Synaptic Plasticity Abnormalities in Fetal Alcohol Spectrum Disorders.Cells. 2023 Jan 29;12(3):442. doi: 10.3390/cells12030442. Cells. 2023. PMID: 36766783 Free PMC article. Review.
-
Fetal Alcohol Spectrum Disorder: Potential Role of Endocannabinoids Signaling.Brain Sci. 2015 Oct 29;5(4):456-93. doi: 10.3390/brainsci5040456. Brain Sci. 2015. PMID: 26529026 Free PMC article. Review.
Cited by
-
Effects of Genetics and Sex on Acute Gene Expression Changes in the Hippocampus Following Neonatal Ethanol Exposure in BXD Recombinant Inbred Mouse Strains.Brain Sci. 2022 Nov 29;12(12):1634. doi: 10.3390/brainsci12121634. Brain Sci. 2022. PMID: 36552094 Free PMC article.
-
Effects of alcohol on the transcriptome, methylome and metabolome of in vitro gastrulating human embryonic cells.Dis Model Mech. 2025 Jun 1;18(6):dmm052150. doi: 10.1242/dmm.052150. Epub 2025 Jun 18. Dis Model Mech. 2025. PMID: 40401629 Free PMC article.
-
Effects on Synaptic Plasticity Markers in Fetal Mice and HT22 Neurons upon F-53B Exposure: The Role of PKA Cytoplasmic Retention.Environ Health (Wash). 2024 Aug 16;2(11):776-785. doi: 10.1021/envhealth.4c00098. eCollection 2024 Nov 15. Environ Health (Wash). 2024. PMID: 39568694 Free PMC article.
-
Utility of the Zebrafish Model for Studying Neuronal and Behavioral Disturbances Induced by Embryonic Exposure to Alcohol, Nicotine, and Cannabis.Cells. 2023 Oct 23;12(20):2505. doi: 10.3390/cells12202505. Cells. 2023. PMID: 37887349 Free PMC article. Review.
-
The interaction of genetic sex and prenatal alcohol exposure on health across the lifespan.Front Neuroendocrinol. 2023 Oct;71:101103. doi: 10.1016/j.yfrne.2023.101103. Epub 2023 Oct 4. Front Neuroendocrinol. 2023. PMID: 37802472 Free PMC article. Review.
References
-
- CDC Alcohol and Pregnancy. [(accessed on 15 February 2022)];2016 Available online: www.cdc.gov/vitalsigns/fasd.
Grants and funding
LinkOut - more resources
Full Text Sources

