Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review
- PMID: 35741711
- PMCID: PMC9222975
- DOI: 10.3390/genes13060949
Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review
Abstract
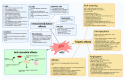
With the insufficient satisfaction rates and high cost of operative treatment for osteoarthritis (OA), alternatives have been sought. Furthermore, the inability of current medications to arrest disease progression has led to rapidly growing clinical research relating to mesenchymal stem cells (MSCs). The availability and function of MSCs vary according to tissue source. The three primary sources include the placenta, bone marrow, and adipose tissue, all of which offer excellent safety profiles. The primary mechanisms of action are trophic and immunomodulatory effects, which prevent the further degradation of joints. However, the function and degree to which benefits are observed vary significantly based on the exosomes secreted by MSCs. Paracrine and autocrine mechanisms prevent cell apoptosis and tissue fibrosis, initiate angiogenesis, and stimulate mitosis via growth factors. MSCs have even been shown to exhibit antimicrobial effects. Clinical results incorporating clinical scores and objective radiological imaging have been promising, but a lack of standardization in isolating MSCs prevents their incorporation in current guidelines.
Keywords: adipose tissue; bone marrow; mesenchymal stem cells; osteoarthritis; placenta; stromal vascular fraction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Comparative Study on the Effect of Exosomes Secreted by Mesenchymal Stem Cells Derived from Adipose and Bone Marrow Tissues in the Treatment of Osteoarthritis-Induced Mouse Model.Biomed Res Int. 2021 Sep 27;2021:9688138. doi: 10.1155/2021/9688138. eCollection 2021. Biomed Res Int. 2021. PMID: 34616850 Free PMC article.
-
Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy.Biomaterials. 2018 Aug;174:67-78. doi: 10.1016/j.biomaterials.2018.04.055. Epub 2018 May 3. Biomaterials. 2018. PMID: 29783118
-
Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis.J Cell Mol Med. 2021 Oct;25(19):9281-9294. doi: 10.1111/jcmm.16860. Epub 2021 Aug 27. J Cell Mol Med. 2021. PMID: 34448527 Free PMC article.
-
Roles of Exosomes from Mesenchymal Stem Cells in Treating Osteoarthritis.Cell Reprogram. 2020 Jun;22(3):107-117. doi: 10.1089/cell.2019.0098. Epub 2020 May 4. Cell Reprogram. 2020. PMID: 32364765 Review.
-
Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis?Stem Cell Res Ther. 2019 Nov 21;10(1):340. doi: 10.1186/s13287-019-1445-0. Stem Cell Res Ther. 2019. PMID: 31753036 Free PMC article. Review.
Cited by
-
Novel Approach in Rectovaginal Fistula Treatment: Combination of Modified Martius Flap and Autologous Micro-Fragmented Adipose Tissue.Biomedicines. 2023 Sep 11;11(9):2509. doi: 10.3390/biomedicines11092509. Biomedicines. 2023. PMID: 37760949 Free PMC article.
-
Mesenchymal Stromal Cells-Derived Extracellular Vesicles as Potential Treatments for Osteoarthritis.Pharmaceutics. 2023 Jun 25;15(7):1814. doi: 10.3390/pharmaceutics15071814. Pharmaceutics. 2023. PMID: 37514001 Free PMC article. Review.
-
Recent advances in mesenchymal stem cell therapy for multiple sclerosis: clinical applications and challenges.Front Cell Dev Biol. 2025 Feb 3;13:1517369. doi: 10.3389/fcell.2025.1517369. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 39963155 Free PMC article. Review.
-
Mesenchymal Stromal Cell Exosome-Induced Vascular Regeneration in a PCOS Mouse Model.Reprod Sci. 2025 Mar;32(3):825-835. doi: 10.1007/s43032-024-01720-7. Epub 2024 Oct 15. Reprod Sci. 2025. PMID: 39407058
-
Stem cell-based therapy for human diseases.Signal Transduct Target Ther. 2022 Aug 6;7(1):272. doi: 10.1038/s41392-022-01134-4. Signal Transduct Target Ther. 2022. PMID: 35933430 Free PMC article. Review.
References
-
- Primorac D., Molnar V., Matišić V., Hudetz D., Jeleč Ž., Rod E., Čukelj F., Vidović D., Vrdoljak T., Dobričić B., et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals. 2021;14:205. doi: 10.3390/ph14030205. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

