Biallelic Optic Atrophy 1 (OPA1) Related Disorder-Case Report and Literature Review
- PMID: 35741767
- PMCID: PMC9223020
- DOI: 10.3390/genes13061005
Biallelic Optic Atrophy 1 (OPA1) Related Disorder-Case Report and Literature Review
Abstract
Dominant optic atrophy (DOA), MIM # 605290, is the most common hereditary optic neuropathy inherited in an autosomal dominant pattern. Clinically, it presents a progressive decrease in vision, central visual field defects, and retinal ganglion cell loss. A biallelic mode of inheritance causes syndromic DOA or Behr phenotype, MIM # 605290. This case report details a family with Biallelic Optic Atrophy 1 (OPA1). The proband is a child with a severe phenotype and two variants in the OPA1 gene. He presented with congenital nystagmus, progressive vision loss, and optic atrophy, as well as progressive ataxia, and was found to have two likely pathogenic variants in his OPA1 gene: c.2287del (p.Ser763Valfs*15) maternally inherited and c.1311A>G (p.lIle437Met) paternally inherited. The first variant is predicted to be pathogenic and likely to cause DOA. In contrast, the second is considered asymptomatic by itself but has been reported in patients with DOA phenotype and is presumed to act as a phenotypic modifier. On follow-up, he developed profound vision impairment, intractable seizures, and metabolic strokes. A literature review of reported biallelic OPA1-related Behr syndrome was performed. Twenty-one cases have been previously reported. All share an early-onset, severe ocular phenotype and systemic features, which seem to be the hallmark of the disease.
Keywords: Behr disease; biallelic OPA1; optic neuropathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


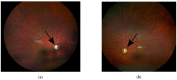
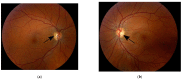




References
-
- le Roux B., Lenaers G., Zanlonghi X., Patrizia A.B., Chabrun F., Foulonneau T., Caignard A., Leruez S., Gohier P., Procaccio V., et al. OPA1: 516 unique variants and 831 patients registered in an updated centralized Variome database. Orphanet J. Rare Dis. 2019;14:124. doi: 10.1186/s13023-019-1187-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

