Risk Measurement of Perinatal and Neonatal Morbidity Characteristics and Applicability of GAIA Case Definitions: Results and Lessons Learnt of a Hospital-Based Prospective Cohort Study in the Valencia Region (2019-2020)
- PMID: 35742384
- PMCID: PMC9223180
- DOI: 10.3390/ijerph19127132
Risk Measurement of Perinatal and Neonatal Morbidity Characteristics and Applicability of GAIA Case Definitions: Results and Lessons Learnt of a Hospital-Based Prospective Cohort Study in the Valencia Region (2019-2020)
Abstract
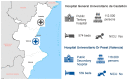
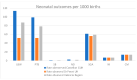
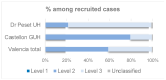
Post-marketing safety surveillance of new vaccines aimed to be administered during pregnancy is crucial to orchestrate efficient adverse events evaluation. This is of special relevance in the current landscape of new vaccines being introduced in the pregnant women population, and particularly due to the recent administration of COVID-19 vaccines in pregnant women. This multi-center prospective cohort study, nested within the WHO-Global Vaccine Safety-MCC study, involved two hospitals in the Valencia region. Hereby, the incidence rates of seven perinatal and neonatal outcomes in the Valencia region are presented. The pooled data analysis of the two Valencian hospitals allowed the estimation of incidence rates in the Valencia Region (per 1000 live births): 86.7 for low birth weight, 78.2 for preterm birth, 58.8 for small for gestational age, 13 for congenital microcephaly, 0.4 for stillbirth, 1.2 for neonatal death and 6.5 for neonatal infection. These figures are in line with what is expected from a high-income country and the previously reported rates for Spain and Europe, except for the significantly increased rate for congenital microcephaly. Regarding the data for maternal immunization, the vaccination status was collected for 94.4% of the screened pregnant women, highlighting the high quality of the Valencian Vaccine Registry. The study also assessed the Valencian hospitals' capacity for identifying and collecting data on maternal immunization status, as well as the applicability of the GAIA definitions to the identified outcomes.
Keywords: incidence rates; maternal immunization; neonatal outcomes; pregnancy; standardized case definitions; vaccination; vaccine safety.
Conflict of interest statement
A.O. has attended several conferences whose registration, travel and accommodation costs have been covered by pharmaceutical companies. A.O. and his institution received research grants to develop observational studies and clinical trials from SP, GSK and MSD related to the vaccines. A.O. also acted as advisor for SP, GSK and MSD for other vaccines. J.D. has received research grants from SP, GSK, Seqirus. J.D. has been a principal investigator in clinical trials with influenza vaccine for GSK, SP, ABBOT, and has acted as an advisor for SP and MSD. Member of Monitoring committee of clinical trials for Janssen and GSK. The rest of the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- WHO WHO Fact Sheet No. 333: Newborns: Reducing Mortality; WHO Official Website. 2016. [(accessed on 20 April 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-morta....
-
- Kochhar S. Harmonized Safety Monitoring of Immunization in Pregnancy—The Global Alignment of Immunization Safety Assessment in Pregnancy (GAIA) Project. 2017: Impatient Optimists. Bill & Melinda Gates Foundation; Seattle, WA, USA: 2017.
-
- Keller-Stanislawski B., Englund J.A., Kang G., Mangtani P., Neuzil K., Nohynek H., Pless R., Lambach P., Zuber P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32:7057–7064. doi: 10.1016/j.vaccine.2014.09.052. - DOI - PubMed
-
- Lackritz E.S.A., Stepanchak M., Englund J., Tavares Da Silva F., Sevene E. Maternal Immunization Safety Monitoring in Low- and Middle-Income Countries: A Roadmap for Program Development. Building an Approach That Is Practical, Affordable, and Sustainable. Global Alliance to Prevent Prematurity and Stillbirth (GAPPS); Lynnwood, WA, USA: 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

