Booster Doses of Anti COVID-19 Vaccines: An Overview of Implementation Policies among OECD and EU Countries
- PMID: 35742479
- PMCID: PMC9222878
- DOI: 10.3390/ijerph19127233
Booster Doses of Anti COVID-19 Vaccines: An Overview of Implementation Policies among OECD and EU Countries
Abstract
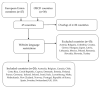
The need for an anti-COVID-19 booster dose posed an organizational challenge for health policy makers worldwide. Therefore, this study aimed to explore the health policies regarding the booster dose through an overview of recommendations issued in high-income countries. Between 10 November and 16 December 2021, the authors searched for state-level official documents about the offer of the booster dose, considering the 43 countries belonging to the European Union (EU) or the Organisation for Economic Co-operation and Development (OECD). Mainly due to the lack of English translation, 15 countries were excluded. A total of 135 documents were selected. Almost all the countries started administering the booster dose between September and November 2021. The most used products were mRNA vaccines, followed by Vaxzevria-AstraZeneca and Jcovden-Janssen/Johnson & Johnson. All countries established criteria to define categories of individuals to be vaccinated as a priority. A six/five-months interval was the main choice for general population vaccinated with mRNA vaccines, while shorter intervals were chosen for vulnerable individuals or other vaccines. Despite diversities related to the differences in health systems, economical resources, and population numbers, and the need to adapt all these factors to a massive vaccination campaign, a progressive convergence towards the same vaccination policies was highlighted.
Keywords: COVID-19; booster dose; health care policy; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
References
-
- Ritchie H., Mathieu E., Rodés-Guirao L., Appel C., Giattino C., Ortiz-Ospina E., Hasell J., Macdonald B., Beltekian D., Roser M. Coronavirus Pandemic (COVID-19). Our World in Data. 2020. [(accessed on 8 January 2022)]. Available online: https://ourworldindata.org/coronavirus.
-
- Fiolet T., Kherabi Y., MacDonald C.-J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical