Using the Dynamic SWOT Analysis to Assess Options for Implementing the HB-HTA Model
- PMID: 35742532
- PMCID: PMC9224318
- DOI: 10.3390/ijerph19127281
Using the Dynamic SWOT Analysis to Assess Options for Implementing the HB-HTA Model
Abstract
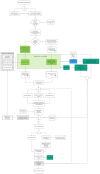
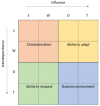
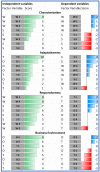
This paper is aimed at exploring the role of the HB-HTA ecosystem as an important pathway for popularizing the implementation of innovations in healthcare organizations. The scientific debate has largely been focused on the rising importance of HB-HTA and the principles guiding the process. Solutions implemented by individual countries differ, which may be rooted in historical, cultural, and institutional differences. Our understanding of the impact of individual countries' healthcare systems on HB-HTA solutions and infrastructure still lacks a basis in interpretative studies. A conceptual framework is proposed to assess the aptness of the HB-HTA model designed for hospitals operating in a country or region, focused on the concepts of adaptiveness and responsiveness to features of the healthcare system present there. A tool is proposed for investigating factors that are likely to assist the successful implementation of the HB-HTA ecosystem. A dynamic SWOT analysis on the case of the HB-HTA model designed for Poland provides interesting insights into the building of the conceptual framework. The results of this study help explain how to create an HB-HTA model that is best adapted to the regional or national healthcare system, including potential risks and opportunities.
Keywords: SWOT analysis; health technology assessment; hospital-based HTA.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Sampietro Colom L., Lach K., Haro I.E., Sroka S., Cicchetti A., Marchetti M., Iacopino V., Kidholm K., Ølholm A.M., Birk Olsen M., et al. The AdHopHTA Handbook: A Handbook of Hospital-Based Health Technology Assessment. 2015. [(accessed on 10 March 2022)]. Available online: http://www.adhophta.eu/sites/files/adhophta/media/adhophta_handbook_webs....
-
- Martelli N., Lelong A.S., Prognon P., Pineau J. Hospital-based health technology assessment for innovative medical devices in university hospitals and the role of hospital pharmacists: Learning from international experience. Int. J. Technol. Assess. Health Care. 2013;29:185–191. doi: 10.1017/S0266462313000019. - DOI - PubMed
-
- Barnieh L., Manns B., Harris A., Blom M., Donaldson C., Klarenbach S., Husereau D., Lorenzetti D., Clement F. A synthesis of drug reimbursement decision-making processes in organisation for economic co-operation and development countries. Value Health. 2014;17:98–108. doi: 10.1016/j.jval.2013.10.008. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

