Lowering n-6/ n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice
- PMID: 35742829
- PMCID: PMC9224551
- DOI: 10.3390/ijms23126384
Lowering n-6/ n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice
Abstract
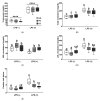
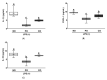
Obesity is closely associated with low-grade chronic and systemic inflammation and dyslipidemia, and the consumption of omega-3 polyunsaturated fatty acids (n-3 PUFAs) may modulate obesity-related disorders, such as inflammation and dyslipidemia. An emerging research question is to understand the dietary intervention strategy that is more important regarding n-3 PUFA consumption: (1) a lower ratio of n-6/n-3 PUFAs or (2) a higher amount of n-3 PUFAs consumption. To understand the desirable dietary intervention method of n-3 PUFAs consumption, we replaced lard from the experimental diets with either perilla oil (PO) or corn oil (CO) to have identical n-3 amounts in the experimental diets. PO had a lower n-6/n-3 ratio, whereas CO contained higher amounts of PUFAs; it inherently contained relatively lower n-3 but higher n-6 PUFAs than PO. After the 12-week dietary intervention in ob/ob mice, dyslipidemia was observed in the normal chow and CO-fed ob/ob mice; however, PO feeding increased the high density lipoprotein-cholesterol (HDL-C) level; further, not only did the HDL-C level increase, the low density lipoprotein-cholesterol (LDL-C) and triglyceride (TG) levels also decreased significantly after lipopolysaccharide (LPS) injection. Consequently, extra TG accumulated in the liver and white adipose tissue (WAT) of normal chow- or CO-fed ob/ob mice after LPS injection; however, PO consumption decreased serum TG accumulation in the liver and WAT. PUFAs replacement attenuated systemic inflammation induced by LPS injection by increasing anti-inflammatory cytokines but inhibiting pro-inflammatory cytokine production in the serum and WAT. PO further decreased hepatic inflammation and fibrosis in comparison with the ND and CO. Hepatic functional biomarkers (aspartate aminotransferase (AST) and alanine transaminase (ALT) levels) were also remarkably decreased in the PO group. In LPS-challenged ob/ob mice, PO and CO decreased adipocyte size and adipokine secretion, with a reduction in phosphorylation of MAPKs compared to the ND group. In addition, LPS-inducible endoplasmic reticulum (ER) and oxidative stress decreased with consumption of PUFAs. Taken together, PUFAs from PO and CO play a role in regulating obesity-related disorders. Moreover, PO, which possesses a lower ratio of n-6/n-3 PUFAs, remarkably alleviated metabolic dysfunction in LPS-induced ob/ob mice. Therefore, an interventional trial considering the ratio of n-6/n-3 PUFAs may be desirable for modulating metabolic complications, such as inflammatory responses and ER stress in the circulation, liver, and/or WAT.
Keywords: cardiac risk factor; dyslipidemia; inflammation; obesity; perilla oil and corn oil; polyunsaturated fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
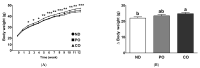
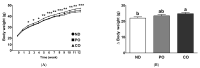













References
-
- Bae J.C., Cho N.H., Suh S., Kim J.H., Hur K.Y., Jin S.M., Lee M.K. Cardiovascular disease incidence, mortality and case fatality related to diabetes and metabolic syndrome: A community-based prospective study (Ansung-Ansan cohort 2001-12) J. Diabetes. 2015;7:791–799. doi: 10.1111/1753-0407.12248. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

