EGFR-Mutated Non-Small Cell Lung Cancer and Resistance to Immunotherapy: Role of the Tumor Microenvironment
- PMID: 35742933
- PMCID: PMC9224267
- DOI: 10.3390/ijms23126489
EGFR-Mutated Non-Small Cell Lung Cancer and Resistance to Immunotherapy: Role of the Tumor Microenvironment
Abstract
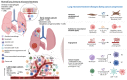
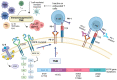
Lung cancer is a leading cause of cancer-related deaths worldwide. About 10-30% of patients with non-small cell lung cancer (NSCLC) harbor mutations of the EGFR gene. The Tumor Microenvironment (TME) of patients with NSCLC harboring EGFR mutations displays peculiar characteristics and may modulate the antitumor immune response. EGFR activation increases PD-L1 expression in tumor cells, inducing T cell apoptosis and immune escape. EGFR-Tyrosine Kinase Inhibitors (TKIs) strengthen MHC class I and II antigen presentation in response to IFN-γ, boost CD8+ T-cells levels and DCs, eliminate FOXP3+ Tregs, inhibit macrophage polarization into the M2 phenotype, and decrease PD-L1 expression in cancer cells. Thus, targeted therapy blocks specific signaling pathways, whereas immunotherapy stimulates the immune system to attack tumor cells evading immune surveillance. A combination of TKIs and immunotherapy may have suboptimal synergistic effects. However, data are controversial because activated EGFR signaling allows NSCLC cells to use multiple strategies to create an immunosuppressive TME, including recruitment of Tumor-Associated Macrophages and Tregs and the production of inhibitory cytokines and metabolites. Therefore, these mechanisms should be characterized and targeted by a combined pharmacological approach that also concerns disease stage, cancer-related inflammation with related systemic symptoms, and the general status of the patients to overcome the single-drug resistance development.
Keywords: EGFR mutations; immunotherapy resistance; non-small cell lung cancer; tumor microenvironment; tumor-associated macrophages; tyrosine kinase inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mansuet-Lupo A., Alifano M., Pécuchet N., Biton J., Becht E., Goc J., Germain C., Ouakrim H., Régnard J.F., Cremer I., et al. Intratumoral immune cell densities are associated with lung adenocarcinoma gene alterations. Am. J. Respir. Crit. Care Med. 2016;194:1403–1412. doi: 10.1164/rccm.201510-2031OC. - DOI - PubMed
-
- Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

