Development and Preliminary Testing of Porcine Blood-Derived Endothelial-like Cells for Vascular Tissue Engineering Applications: Protocol Optimisation and Seeding of Decellularised Human Saphenous Veins
- PMID: 35743073
- PMCID: PMC9223800
- DOI: 10.3390/ijms23126633
Development and Preliminary Testing of Porcine Blood-Derived Endothelial-like Cells for Vascular Tissue Engineering Applications: Protocol Optimisation and Seeding of Decellularised Human Saphenous Veins
Abstract
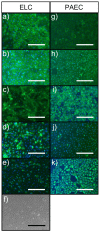
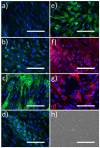
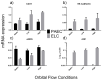
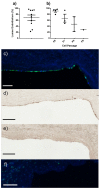
Functional endothelial cells (EC) are a critical interface between blood vessels and the thrombogenic flowing blood. Disruption of this layer can lead to early thrombosis, inflammation, vessel restenosis, and, following coronary (CABG) or peripheral (PABG) artery bypass graft surgery, vein graft failure. Blood-derived ECs have shown potential for vascular tissue engineering applications. Here, we show the development and preliminary testing of a method for deriving porcine endothelial-like cells from blood obtained under clinical conditions for use in translational research. The derived cells show cobblestone morphology and expression of EC markers, similar to those seen in isolated porcine aortic ECs (PAEC), and when exposed to increasing shear stress, they remain viable and show mRNA expression of EC markers similar to PAEC. In addition, we confirm the feasibility of seeding endothelial-like cells onto a decellularised human vein scaffold with approximately 90% lumen coverage at lower passages, and show that increasing cell passage results in reduced endothelial coverage.
Keywords: bioengineering; cell seeding; endothelial colony forming cells; endothelium; vascular graft.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Samano N., Souza D., Pinheiro B.B., Kopjar T., Dashwood M. Twenty-Five Years of No-Touch Saphenous Vein Harvesting for Coronary Artery Bypass Grafting: Structural Observations and Impact on Graft Performance. Braz. J. Cardiovasc. Surg. 2020;35:91–99. doi: 10.21470/1678-9741-2019-0238. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- PG/104/32652/BHF_/British Heart Foundation/United Kingdom
- NC/N003268/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- IG/14/2/30991/BHF_/British Heart Foundation/United Kingdom
- FS/18/1/33234/BHF_/British Heart Foundation/United Kingdom
- MR/L012723/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

