Cationic Surfactants as Disinfectants against SARS-CoV-2
- PMID: 35743090
- PMCID: PMC9224290
- DOI: 10.3390/ijms23126645
Cationic Surfactants as Disinfectants against SARS-CoV-2
Abstract
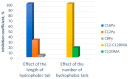
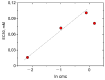
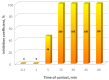
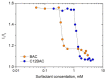
The virucidal activity of a series of cationic surfactants differing in the length and number of hydrophobic tails (at the same hydrophilic head) and the structure of the hydrophilic head (at the same length of the hydrophobic n-alkyl tail) was compared. It was shown that an increase in the length and number of hydrophobic tails, as well as the presence of a benzene ring in the surfactant molecule, enhance the virucidal activity of the surfactant against SARS-CoV-2. This may be due to the more pronounced ability of such surfactants to penetrate and destroy the phospholipid membrane of the virus. Among the cationic surfactants studied, didodecyldimethylammonium bromide was shown to be the most efficient as a disinfectant, its 50% effective concentration (EC50) being equal to 0.016 mM. Two surfactants (didodecyldimethylammonium bromide and benzalkonium chloride) can deactivate SARS-CoV-2 in as little as 5 s.
Keywords: COVID-19; SARS-CoV-2; cationic surfactants; disinfectants; quaternary ammonium compounds; virucidal activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Virucidal Coatings Active Against SARS-CoV-2.Molecules. 2024 Oct 20;29(20):4961. doi: 10.3390/molecules29204961. Molecules. 2024. PMID: 39459329 Free PMC article.
-
Evaluating the virucidal activity of four disinfectants against SARS-CoV-2.Am J Infect Control. 2022 Mar;50(3):319-324. doi: 10.1016/j.ajic.2021.10.035. Epub 2021 Nov 12. Am J Infect Control. 2022. PMID: 34774899 Free PMC article.
-
Comparative efficacy evaluation of disinfectants against severe acute respiratory syndrome coronavirus-2.J Hosp Infect. 2023 Jan;131:12-22. doi: 10.1016/j.jhin.2022.09.011. Epub 2022 Sep 30. J Hosp Infect. 2023. PMID: 36183929 Free PMC article.
-
Assessment of ecological hazards and environmental fate of disinfectant quaternary ammonium compounds.Ecotoxicol Environ Saf. 2020 Dec 15;206:111116. doi: 10.1016/j.ecoenv.2020.111116. Epub 2020 Sep 2. Ecotoxicol Environ Saf. 2020. PMID: 32890921 Free PMC article. Review.
-
Disinfectants against SARS-CoV-2: A Review.Viruses. 2022 Aug 4;14(8):1721. doi: 10.3390/v14081721. Viruses. 2022. PMID: 36016342 Free PMC article. Review.
Cited by
-
Study of Cationic Surfactants Raw Materials for COVID-19 Disinfecting Formulations by Potentiometric Surfactant Sensor.Sensors (Basel). 2023 Feb 13;23(4):2126. doi: 10.3390/s23042126. Sensors (Basel). 2023. PMID: 36850724 Free PMC article.
-
Assessment of Strategies for Preserving Swine Viral RNA Targets in Diagnostic Specimens.Microorganisms. 2024 Feb 18;12(2):410. doi: 10.3390/microorganisms12020410. Microorganisms. 2024. PMID: 38399814 Free PMC article. Review.
-
Double Photocrosslinked Responsive Hydrogels Based on Hydroxypropyl Guar.Int J Mol Sci. 2023 Dec 14;24(24):17477. doi: 10.3390/ijms242417477. Int J Mol Sci. 2023. PMID: 38139305 Free PMC article.
-
Cyclic lipopeptides as membrane fusion inhibitors against SARS-CoV-2: New tricks for old dogs.Antiviral Res. 2023 Apr;212:105575. doi: 10.1016/j.antiviral.2023.105575. Epub 2023 Mar 2. Antiviral Res. 2023. PMID: 36868316 Free PMC article.
-
In vitro evaluation for estrogenic mechanisms of the disinfectant benzalkonium chloride as an emerging contaminant.Braz J Med Biol Res. 2023 Jul 21;56:e12784. doi: 10.1590/1414-431X2023e12784. eCollection 2023. Braz J Med Biol Res. 2023. PMID: 37493774 Free PMC article.
References
-
- Worldometer Coronavirus. [(accessed on 30 May 2022)]. Available online: https://www.worldometers.info/coronavirus/#countries.
-
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

