Cancer Malignancy Is Correlated with Upregulation of PCYT2-Mediated Glycerol Phosphate Modification of α-Dystroglycan
- PMID: 35743105
- PMCID: PMC9223686
- DOI: 10.3390/ijms23126662
Cancer Malignancy Is Correlated with Upregulation of PCYT2-Mediated Glycerol Phosphate Modification of α-Dystroglycan
Abstract
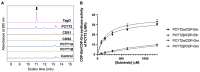
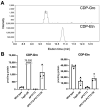
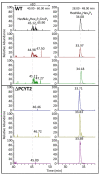
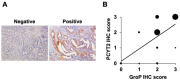
The dystrophin-glycoprotein complex connects the cytoskeleton with base membrane components such as laminin through unique O-glycans displayed on α-dystroglycan (α-DG). Genetic impairment of elongation of these glycans causes congenital muscular dystrophies. We previously identified that glycerol phosphate (GroP) can cap the core part of the α-DG O-glycans and terminate their further elongation. This study examined the possible roles of the GroP modification in cancer malignancy, focusing on colorectal cancer. We found that the GroP modification critically depends on PCYT2, which serves as cytidine 5'-diphosphate-glycerol (CDP-Gro) synthase. Furthermore, we identified a significant positive correlation between cancer progression and GroP modification, which also correlated positively with PCYT2 expression. Moreover, we demonstrate that GroP modification promotes the migration of cancer cells. Based on these findings, we propose that the GroP modification by PCYT2 disrupts the glycan-mediated cell adhesion to the extracellular matrix and thereby enhances cancer metastasis. Thus, the present study suggests the possibility of novel approaches for cancer treatment by targeting the PCYT2-mediated GroP modification.
Keywords: CDP-glycerol; PCYT2; cancer malignancy; glycerol phosphate modification; matriglycan; α-dystroglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
PCYT2 synthesizes CDP-glycerol in mammals and reduced PCYT2 enhances the expression of functionally glycosylated α-dystroglycan.J Biochem. 2021 Oct 11;170(2):183-194. doi: 10.1093/jb/mvab069. J Biochem. 2021. PMID: 34255834
-
CDP-glycerol inhibits the synthesis of the functional O-mannosyl glycan of α-dystroglycan.J Biol Chem. 2018 Aug 3;293(31):12186-12198. doi: 10.1074/jbc.RA118.003197. Epub 2018 Jun 8. J Biol Chem. 2018. PMID: 29884773 Free PMC article.
-
Biosynthetic Mechanisms and Biological Significance of Glycerol Phosphate-Containing Glycan in Mammals.Molecules. 2021 Nov 4;26(21):6675. doi: 10.3390/molecules26216675. Molecules. 2021. PMID: 34771084 Free PMC article. Review.
-
Inhibitory machinery for the functional dystroglycan glycosylation.J Biochem. 2023 Apr 26;173(5):333-335. doi: 10.1093/jb/mvad003. J Biochem. 2023. PMID: 36760122
-
Aberrant glycosylation of alpha-dystroglycan and congenital muscular dystrophies.Acta Myol. 2005 Oct;24(2):64-9. Acta Myol. 2005. PMID: 16550917 Review.
Cited by
-
Adding metabolic tasks to human GEM models to improve the study of gene targets and their associated toxicities.Sci Rep. 2024 Jul 27;14(1):17265. doi: 10.1038/s41598-024-68073-8. Sci Rep. 2024. PMID: 39068208 Free PMC article.
-
Medwakh smoking induces alterations in salivary proteins and cytokine expression: a clinical exploratory proteomics investigation.Clin Proteomics. 2025 Jan 17;22(1):2. doi: 10.1186/s12014-024-09520-6. Clin Proteomics. 2025. PMID: 39819313 Free PMC article.
-
PCYT2 mediates ovarian epithelial cancer metastasis by regulating cell membrane fluidity through the AMPK/FOXO1 signalling pathway.Sci Rep. 2025 Apr 8;15(1):12044. doi: 10.1038/s41598-025-96405-9. Sci Rep. 2025. PMID: 40200068 Free PMC article.
-
PCYT2 overexpression induces mitochondrial damage and promotes apoptosis in hepatocellular carcinoma cells.PLoS One. 2025 May 28;20(5):e0323974. doi: 10.1371/journal.pone.0323974. eCollection 2025. PLoS One. 2025. PMID: 40434993 Free PMC article.
-
Role of Post-Translational Modifications in Colorectal Cancer Metastasis.Cancers (Basel). 2024 Feb 3;16(3):652. doi: 10.3390/cancers16030652. Cancers (Basel). 2024. PMID: 38339403 Free PMC article. Review.
References
-
- Hara Y., Kanagawa M., Kunz S., Yoshida-Moriguchi T., Satz J.S., Kobayashi Y.M., Zhu Z., Burden S.J., Oldstone M.B.A., Campbell K.P. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl. Acad. Sci. USA. 2011;108:17426–17431. doi: 10.1073/pnas.1114836108. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

