The Effect of High and Variable Glucose on the Viability of Endothelial Cells Co-Cultured with Smooth Muscle Cells
- PMID: 35743147
- PMCID: PMC9223437
- DOI: 10.3390/ijms23126704
The Effect of High and Variable Glucose on the Viability of Endothelial Cells Co-Cultured with Smooth Muscle Cells
Abstract
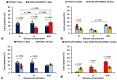
Diabetes mellitus causes endothelial dysfunction. The aim of this study was to investigate the effect of normal (5 mmol/L), high (20 mmol/L), and fluctuating (5 and 20 mmol/L changed every day) glucose concentration in the culture medium on the viability of human umbilical vein endothelial cells (HUVECs) co-cultured with human umbilical artery smooth muscle cells (HUASMCs). The cultures were conducted on semi-permeable flat polysulfone (PSU) fibronectin-coated membranes immobilized in self-made inserts. The insert contained either HUVECs on a single membrane or HUASMCs and HUVECs on two membranes close to each other. Cultures were conducted for 7 or 14 days. Apoptosis, mitochondrial potential, and the production of reactive oxygen species and lactate by HUVECs were investigated. The results indicate that fluctuations in glucose concentration have a stronger negative effect on HUVECs viability than constant high glucose concentration. High and fluctuating glucose concentrations slow down cell proliferation compared to the culture carried out in the medium with normal glucose concentration. In conclusion, HUASMCs affect the viability of HUVECs when both types of cells are co-cultured in medium with normal or variable glucose concentration.
Keywords: DM; HUASMCs; HUVECs; co-culture; diabetes; diabetes complications; endothelial cells; high concentration of glucose; reactive oxygen species; smooth muscle cells; variable concentration of glucose; viability.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- International Diabetes Federation . IDF Diabetes Atlas. 9th ed. International Diabetes Federation; Brussels, Belgium: 2019.
-
- Ciechanowska A., Gora I., Sabalinska S., Foltynski P., Ladyzynski P. Effect of glucose concentration and culture substrate on HUVECs viability in in vitro cultures: A literature review and own results. Biocybern. Biomed. Eng. 2021;41:1390–1405. doi: 10.1016/j.bbe.2021.04.010. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

