NMDA and P2X7 Receptors Require Pannexin 1 Activation to Initiate and Maintain Nociceptive Signaling in the Spinal Cord of Neuropathic Rats
- PMID: 35743148
- PMCID: PMC9223805
- DOI: 10.3390/ijms23126705
NMDA and P2X7 Receptors Require Pannexin 1 Activation to Initiate and Maintain Nociceptive Signaling in the Spinal Cord of Neuropathic Rats
Abstract
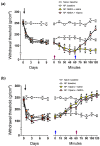
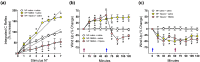
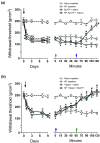
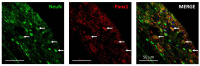
Pannexin 1 (Panx1) is involved in the spinal central sensitization process in rats with neuropathic pain, but its interaction with well-known, pain-related, ligand-dependent receptors, such as NMDA receptors (NMDAR) and P2X7 purinoceptors (P2X7R), remains largely unexplored. Here, we studied whether NMDAR- and P2X7R-dependent nociceptive signaling in neuropathic rats require the activation of Panx1 channels to generate spinal central sensitization, as assessed by behavioral (mechanical hyperalgesia) and electrophysiological (C-reflex wind-up potentiation) indexes. Administration of either a selective NMDAR agonist i.t. (NMDA, 2 mM) or a P2X7R agonist (BzATP, 150 μM) significantly increased both the mechanical hyperalgesia and the C-reflex wind-up potentiation, effects that were rapidly reversed (minutes) by i.t. administration of a selective pannexin 1 antagonist (10panx peptide, 300 μM), with the scores even reaching values of rats without neuropathy. Accordingly, 300 μM 10panx completely prevented the effects of NMDA and BzATP administered 1 h later, on mechanical hyperalgesia and C-reflex wind-up potentiation. Confocal immunofluorescence imaging revealed coexpression of Panx1 with NeuN protein in intrinsic dorsal horn neurons of neuropathic rats. The results indicate that both NMDAR- and P2X7R-mediated increases in mechanical hyperalgesia and C-reflex wind-up potentiation require neuronal Panx1 channel activation to initiate and maintain nociceptive signaling in neuropathic rats.
Keywords: NMDA receptor; P2X7 receptor; neuropathic pain; pannexin 1; wind-up.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
N-methyl-D-aspartate receptor activation is downstream coupled to pannexin 1 opening by Src kinase in dorsal horn neurons: an essential link for mechanical hyperalgesia in nerve-injured rats.Pain. 2025 Jun 1;166(6):1369-1381. doi: 10.1097/j.pain.0000000000003476. Epub 2024 Dec 3. Pain. 2025. PMID: 39630026 Free PMC article.
-
Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord.Pain. 2014 Oct;155(10):2108-15. doi: 10.1016/j.pain.2014.07.024. Epub 2014 Aug 4. Pain. 2014. PMID: 25102401
-
Interactions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: Possible role on chronic pain.Pharmacol Res. 2015 Nov;101:86-93. doi: 10.1016/j.phrs.2015.07.016. Epub 2015 Jul 23. Pharmacol Res. 2015. PMID: 26211949 Review.
-
Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain.Exp Neurol. 2009 Feb;215(2):298-307. doi: 10.1016/j.expneurol.2008.10.018. Epub 2008 Nov 12. Exp Neurol. 2009. PMID: 19046970
-
Pannexin-1 as a potentiator of ligand-gated receptor signaling.Channels (Austin). 2014;8(2):118-23. doi: 10.4161/chan.27978. Epub 2014 Feb 27. Channels (Austin). 2014. PMID: 24576994 Free PMC article. Review.
Cited by
-
The effect of P2X7 antagonism on subcortical spread of optogenetically-triggered cortical spreading depression and neuroinflammation.J Headache Pain. 2024 Jul 24;25(1):120. doi: 10.1186/s10194-024-01807-1. J Headache Pain. 2024. PMID: 39044141 Free PMC article.
-
Persistent transgene expression in peripheral tissues one year post intravenous and intramuscular administration of AAV vectors containing the alphaherpesvirus latency-associated promoter 2.Front Virol. 2024;4:1379991. doi: 10.3389/fviro.2024.1379991. Epub 2024 Mar 28. Front Virol. 2024. PMID: 38665693 Free PMC article.
-
Targeting Pannexin-1 Channels: Addressing the 'Gap' in Chronic Pain.CNS Drugs. 2024 Feb;38(2):77-91. doi: 10.1007/s40263-024-01061-8. Epub 2024 Feb 14. CNS Drugs. 2024. PMID: 38353876 Review.
-
The Pannexin 1 Channel and the P2X7 Receptor Are in Complex Interplay to Regulate the Release of Soluble Ectonucleotidases in the Murine Bladder Lamina Propria.Int J Mol Sci. 2023 Jun 9;24(12):9964. doi: 10.3390/ijms24129964. Int J Mol Sci. 2023. PMID: 37373111 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

