Overexpression of Neurogenin 1 Negatively Regulates Osteoclast and Osteoblast Differentiation
- PMID: 35743149
- PMCID: PMC9223505
- DOI: 10.3390/ijms23126708
Overexpression of Neurogenin 1 Negatively Regulates Osteoclast and Osteoblast Differentiation
Abstract
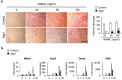
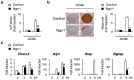
Neurogenin 1 (Ngn1) belongs to the basic helix-loop-helix (bHLH) transcription factor family and plays important roles in specifying neuronal differentiation. The present study aimed to determine whether forced Ngn1 expression contributes to bone homeostasis. Ngn1 inhibited the p300/CREB-binding protein-associated factor (PCAF)-induced acetylation of nuclear factor of activated T cells 1 (NFATc1) and runt-related transcription factor 2 (Runx2) through binding to PCAF, which led to the inhibition of osteoclast and osteoblast differentiation, respectively. In addition, Ngn1 overexpression inhibited the TNF-α- and IL-17A-mediated enhancement of osteoclast differentiation and IL-17A-induced osteoblast differentiation. These findings indicate that Ngn1 can serve as a novel therapeutic agent for treating ankylosing spondylitis with abnormally increased bone formation and resorption.
Keywords: Neurogenin 1; PCAF; ankylosing spondylitis; osteoblast; osteoclast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

