Action Mechanisms of Effectors in Plant-Pathogen Interaction
- PMID: 35743201
- PMCID: PMC9224169
- DOI: 10.3390/ijms23126758
Action Mechanisms of Effectors in Plant-Pathogen Interaction
Abstract
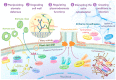
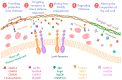
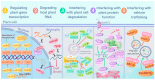
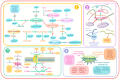
Plant pathogens are one of the main factors hindering the breeding of cash crops. Pathogens, including oomycetes, fungus, and bacteria, secrete effectors as invasion weapons to successfully invade and propagate in host plants. Here, we review recent advances made in the field of plant-pathogen interaction models and the action mechanisms of phytopathogenic effectors. The review illustrates how effectors from different species use similar and distinct strategies to infect host plants. We classify the main action mechanisms of effectors in plant-pathogen interactions according to the infestation process: targeting physical barriers for disruption, creating conditions conducive to infestation, protecting or masking themselves, interfering with host cell physiological activity, and manipulating plant downstream immune responses. The investigation of the functioning of plant pathogen effectors contributes to improved understanding of the molecular mechanisms of plant-pathogen interactions. This understanding has important theoretical value and is of practical significance in plant pathology and disease resistance genetics and breeding.
Keywords: effector; infestation process; pathogen; plant immunity; virulence promotion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ghosh S., Malukani K.K., Chandan R.K., Sonti R.V., Jha G. How Plants Respond to Pathogen Attack: Interaction and Communication. In: Sopory S., editor. Sensory Biology of Plants. Springer; Singapore: 2019. pp. 537–568.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

