Production and Characterization of Peptide Antibodies to the C-Terminal of Frameshifted Calreticulin Associated with Myeloproliferative Diseases
- PMID: 35743246
- PMCID: PMC9223637
- DOI: 10.3390/ijms23126803
Production and Characterization of Peptide Antibodies to the C-Terminal of Frameshifted Calreticulin Associated with Myeloproliferative Diseases
Abstract
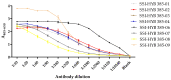
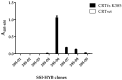
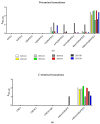
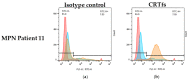
Myeloproliferative Neoplasms (MPNs) constitute a group of rare blood cancers that are characterized by mutations in bone marrow stem cells leading to the overproduction of erythrocytes, leukocytes, and thrombocytes. Mutations in calreticulin (CRT) genes may initiate MPNs, causing a novel variable polybasic stretch terminating in a common C-terminal sequence in the frameshifted CRT (CRTfs) proteins. Peptide antibodies to the mutated C-terminal are important reagents for research in the molecular mechanisms of MPNs and for the development of new diagnostic assays and therapies. In this study, eight peptide antibodies targeting the C-terminal of CRTfs were produced and characterised by modified enzyme-linked immunosorbent assays using resin-bound peptides. The antibodies reacted to two epitopes: CREACLQGWTE for SSI-HYB 385-01, 385-02, 385-03, 385-04, 385-07, 385-08, and 385-09 and CLQGWT for SSI-HYB 385-06. For the majority of antibodies, the residues Cys1, Trp9, and Glu11 were essential for reactivity. SSI-HYB 385-06, with the highest affinity, recognised recombinant CRTfs produced in yeast and the MARIMO cell line expressing CRTfs when examined in Western immunoblotting. Moreover, SSI-HYB 385-06 occasionally reacted to CRTfs from MPN patients when analysed by flow cytometry. The characterized antibodies may be used to understand the role of CRTfs in the pathogenesis of MPNs and to design and develop new diagnostic assays and therapeutic targets.
Keywords: calreticulin; epitope mapping; frameshift mutations; myeloproliferative neoplasms; peptide antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Oncogenic Drivers in Myeloproliferative Neoplasms: From JAK2 to Calreticulin Mutations.Curr Hematol Malig Rep. 2015 Dec;10(4):335-43. doi: 10.1007/s11899-015-0278-x. Curr Hematol Malig Rep. 2015. PMID: 26370832 Review.
-
Selective targeting of mutated calreticulin by the monoclonal antibody INCA033989 inhibits oncogenic function of MPN.Blood. 2024 Nov 28;144(22):2336-2348. doi: 10.1182/blood.2024024373. Blood. 2024. PMID: 39255409
-
CALR frameshift mutations in MPN patient-derived iPSCs accelerate maturation of megakaryocytes.Stem Cell Reports. 2021 Nov 9;16(11):2768-2783. doi: 10.1016/j.stemcr.2021.09.019. Epub 2021 Oct 21. Stem Cell Reports. 2021. PMID: 34678208 Free PMC article.
-
Somatic mutations of calreticulin in myeloproliferative neoplasms.Int J Hematol. 2017 Jun;105(6):743-747. doi: 10.1007/s12185-017-2246-9. Epub 2017 May 3. Int J Hematol. 2017. PMID: 28470469 Review.
-
Overview of Transgenic Mouse Models of Myeloproliferative Neoplasms (MPNs).Curr Protoc Pharmacol. 2017 Jun 22;77:14.40.1-14.40.19. doi: 10.1002/cpph.23. Curr Protoc Pharmacol. 2017. PMID: 28640953 Free PMC article. Review.
Cited by
-
Determination of crucial epitopes in the sperm protein calsperin employing synthetic peptides and monoclonal antibodies.J Pept Sci. 2023 Feb;29(2):e3450. doi: 10.1002/psc.3450. Epub 2022 Oct 2. J Pept Sci. 2023. PMID: 36082776 Free PMC article.
-
Design, Production, Characterization, and Use of Peptide Antibodies.Antibodies (Basel). 2023 Jan 13;12(1):6. doi: 10.3390/antib12010006. Antibodies (Basel). 2023. PMID: 36648890 Free PMC article.
-
Mutant Calreticulin in MPN: Mechanistic Insights and Therapeutic Implications.Curr Hematol Malig Rep. 2025 Jan 8;20(1):4. doi: 10.1007/s11899-024-00749-4. Curr Hematol Malig Rep. 2025. PMID: 39775969 Free PMC article. Review.
-
Fmoc Solid-Phase Peptide Synthesis.Methods Mol Biol. 2024;2821:33-55. doi: 10.1007/978-1-0716-3914-6_3. Methods Mol Biol. 2024. PMID: 38997478
-
Proteogenetic drug response profiling elucidates targetable vulnerabilities of myelofibrosis.Nat Commun. 2023 Oct 12;14(1):6414. doi: 10.1038/s41467-023-42101-z. Nat Commun. 2023. PMID: 37828014 Free PMC article.
References
-
- Coltro G., Loscocco G.G., Vannucchi A.M. Classical Philadelphia-negative myeloproliferative neoplasms (MPNs): A continuum of different disease entities. Int. Rev. Cell Mol. Biol. 2021;365:1–69. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

