Elevated Alpha 1(I) to Alpha 2(I) Collagen Ratio in Dermal Fibroblasts Possibly Contributes to Fibrosis in Systemic Sclerosis
- PMID: 35743254
- PMCID: PMC9224560
- DOI: 10.3390/ijms23126811
Elevated Alpha 1(I) to Alpha 2(I) Collagen Ratio in Dermal Fibroblasts Possibly Contributes to Fibrosis in Systemic Sclerosis
Abstract
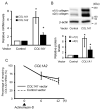
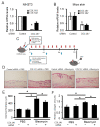
Systemic sclerosis (SSc) is characterized by excessive collagen deposition in the skin and internal organs. Activated fibroblasts are the key effector cells for the overproduction of type I collagen, which comprises the α1(I) and α2(I) chains encoded by COL1A1 and COL1A2, respectively. In this study, we examined the expression patterns of α1(I) and α2(I) collagen in SSc fibroblasts, as well as their co-regulation with each other. The relative expression ratio of COL1A1 to COL1A2 in SSc fibroblasts was significantly higher than that in control fibroblasts. The same result was observed for type I collagen protein levels, indicating that α2(I) collagen is more elevated than α2(I) collagen. Inhibition or overexpression of α1(I) collagen in control fibroblasts affected the α2(I) collagen levels, suggesting that α1(I) collagen might act as an upstream regulator of α2(I) collagen. The local injection of COL1A1 small interfering RNA in a bleomycin-induced SSc mouse model was found to attenuate skin fibrosis. Overall, our data indicate that α2(I) collagen is a potent regulator of type I collagen in SSc; further investigations of the overall regulatory mechanisms of type I collagen may help understand the aberrant collagen metabolism in SSc.
Keywords: extracellular matrix; fibroblast; fibrosis; metabolism; systemic sclerosis; type I collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Roberts A.B., Sporn M.B., Assoian R.K., Smith J.M., Roche N.S., Wakefield L.M., Heine U.I., Liotta L.A., Falanga V., Kehrl J.H., et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

