MCD Diet Rat Model Induces Alterations in Zinc and Iron during NAFLD Progression from Steatosis to Steatohepatitis
- PMID: 35743260
- PMCID: PMC9224179
- DOI: 10.3390/ijms23126817
MCD Diet Rat Model Induces Alterations in Zinc and Iron during NAFLD Progression from Steatosis to Steatohepatitis
Abstract
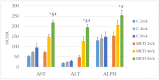
We evaluate the effects of the methionine-choline-deficient (MCD) diet on serum and hepatic zinc (Zn) and iron (Fe) and their relationships with matrix metalloproteinases (MMPs) and their modulators (TIMPs and RECK) as well as hepatic fatty acids using male Wistar rats fed 2-, 4- and 8-week MCD diets. Serum and hepatic Zn decrease after an 8-week MCD diet. Serum Fe increases after an 8-week MCD diet and the same occurs for hepatic Fe. An increase in hepatic MMP activity, associated with a decrease in RECK and TIMPs, is found in the MCD 8-week group. Liver Fe shows a positive correlation versus MMPs and RECK, and an inverse correlation versus TIMPs. A positive correlation is found comparing liver Zn with stearic, vaccenic and arachidonic acids, and an inverse correlation is found with linolenic and docosatetraenoic acids. An opposite trend is found between liver Fe versus these fatty acids. During NAFLD progression from steatosis to steatohepatitis, MCD rats exhibit an increase in Zn and a decrease in Fe levels both in serum and tissue associated with alterations in hepatic MMPs and their inhibitors, and fatty acids. The correlations detected between Zn and Fe versus extracellular matrix modulators and fatty acids support their potential role as therapeutic targets.
Keywords: Fe; MMPs; NAFLD; NASH; RECK; Zn; iron; liver; steatohepatitis; steatosis; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Asprouli E., Kalafati I.P., Sakellari A., Karavoltsos S., Vlachogiannakos J., Revenas K., Kokkinos A., Dassenakis M., Dedoussis G.V., Kalogeropoulos N. Evaluation of Plasma Trace Elements in Different Stages of Nonalcoholic Fatty Liver Disease. Biol. Trace Elem. Res. 2019;188:326–333. doi: 10.1007/s12011-018-1432-9. - DOI - PubMed
-
- George D.K., Goldwurm S., Macdonald G.A., Cowley L.L., Walker N.I., Ward P.J., Jazwinska E.C., Powell L.W. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/S0016-5085(98)70482-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

