Microtube Array Membrane Encapsulated Cell Therapy: A Novel Platform Technology Solution for Treatment of Alzheimer's Disease
- PMID: 35743295
- PMCID: PMC9224941
- DOI: 10.3390/ijms23126855
Microtube Array Membrane Encapsulated Cell Therapy: A Novel Platform Technology Solution for Treatment of Alzheimer's Disease
Abstract
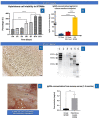
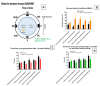
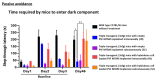
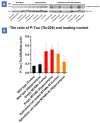
Alzheimer's disease is the most frequent form of dementia in aging population and is presently the world's sixth largest cause of mortality. With the advancement of therapies, several solutions have been developed such as passive immunotherapy against these misfolded proteins, thereby resulting in the clearance. Within this segment, encapsulated cell therapy (ECT) solutions that utilize antibody releasing cells have been proposed with a multitude of techniques under development. Hence, in this study, we utilized our novel and patented Microtube Array Membranes (MTAMs) as an encapsulating platform system with anti-pTau antibody-secreting hybridoma cells to study the impact of it on Alzheimer's disease. In vivo results revealed that in the water maze, the mice implanted with hybridoma cell MTAMs intracranially (IN) and subcutaneously (SC) showed improvement in the time spent the goal quadrant and escape latency. In passive avoidance, hybridoma cell loaded MTAMs (IN and SC) performed significantly well in step-through latency. At the end of treatment, animals with hybridoma cell loaded MTAMs had lower phosphorylated tau (pTau) expression than empty MTAMs had. Combining both experimental results unveiled that the clearance of phosphorylated tau might rescue the cognitive impairment associated with AD.
Keywords: Alzheimer’s disease (AD); encapsulated cell therapy; microtube array membrane (MTAMs); neurodegenerative disease; passive immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Review of electrospun microtube array membrane (MTAM)-a novel new class of hollow fiber for encapsulated cell therapy (ECT) in clinical applications.J Biomed Mater Res B Appl Biomater. 2024 Jan;112(1):e35348. doi: 10.1002/jbm.b.35348. J Biomed Mater Res B Appl Biomater. 2024. PMID: 38247238 Review.
-
Microtube Array Membrane (MTAM)-Based Encapsulated Cell Therapy for Cancer Treatment.Membranes (Basel). 2020 Apr 26;10(5):80. doi: 10.3390/membranes10050080. Membranes (Basel). 2020. PMID: 32357523 Free PMC article.
-
Tau passive immunization inhibits not only tau but also Aβ pathology.Alzheimers Res Ther. 2017 Jan 10;9(1):1. doi: 10.1186/s13195-016-0227-5. Alzheimers Res Ther. 2017. PMID: 28073379 Free PMC article.
-
Cognitive Decline in Preclinical Alzheimer's Disease: Amyloid-Beta versus Tauopathy.J Alzheimers Dis. 2018;61(1):265-281. doi: 10.3233/JAD-170490. J Alzheimers Dis. 2018. PMID: 29154274 Free PMC article. Review.
-
Antibody-free measurement of cerebrospinal fluid tau phosphorylation across the Alzheimer's disease continuum.Mol Neurodegener. 2022 Dec 12;17(1):81. doi: 10.1186/s13024-022-00586-0. Mol Neurodegener. 2022. PMID: 36510321 Free PMC article.
Cited by
-
A versatile genomic transgenesis platform with enhanced λ integrase for human Expi293F cells.Front Bioeng Biotechnol. 2023 Jun 22;11:1198465. doi: 10.3389/fbioe.2023.1198465. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37425360 Free PMC article.
-
Cell primitive-based biomimetic nanomaterials for Alzheimer's disease targeting and therapy.Mater Today Bio. 2023 Sep 1;22:100789. doi: 10.1016/j.mtbio.2023.100789. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37706205 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

