Roles of AGD2a in Plant Development and Microbial Interactions of Lotus japonicus
- PMID: 35743304
- PMCID: PMC9224730
- DOI: 10.3390/ijms23126863
Roles of AGD2a in Plant Development and Microbial Interactions of Lotus japonicus
Abstract
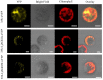
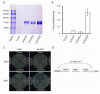
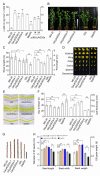
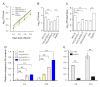
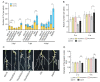
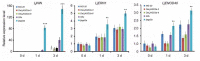
Arabidopsis AGD2 (Aberrant Growth and Death2) and its close homolog ALD1 (AGD2-like defense response protein 1) have divergent roles in plant defense. We previously reported that modulation of salicylic acid (SA) contents by ALD1 affects numbers of nodules produced by Lotus japonicus, but AGD2's role in leguminous plants remains unclear. A combination of enzymatic analysis and biological characterization of genetic materials was used to study the function of AGD2 (LjAGD2a and LjAGD2b) in L. japonicus. Both LjAGD2a and LjAGD2b could complement dapD and dapE mutants of Escherichia coli and had aminotransferase activity in vitro. ljagd2 plants, with insertional mutations of LjAGD2, had delayed flowering times and reduced seed weights. In contrast, overexpression of LjAGD2a in L. japonicus induced early flowering, with increases in seed and flower sizes, but reductions in pollen fertility and seed setting rates. Additionally, ljagd2a mutation resulted in increased expression of nodulin genes and corresponding increases in infection threads and nodule numbers following inoculation with Rhizobium. Changes in expression of LjAGD2a in L. japonicus also affected endogenous SA contents and hence resistance to pathogens. Our results indicate that LjAGD2a functions as an LL-DAP aminotransferase and plays important roles in plant development. Moreover, LjAGD2a activates defense signaling via the Lys synthesis pathway, thereby participating in legume-microbe interaction.
Keywords: Lotus japonicus; development; disease resistance; nodulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Knockdown of LjALD1, AGD2-like defense response protein 1, influences plant growth and nodulation in Lotus japonicus.J Integr Plant Biol. 2014 Nov;56(11):1034-41. doi: 10.1111/jipb.12211. Epub 2014 Jun 19. J Integr Plant Biol. 2014. PMID: 24797909
-
The Phenylalanine Ammonia Lyase Gene LjPAL1 Is Involved in Plant Defense Responses to Pathogens and Plays Diverse Roles in Lotus japonicus-Rhizobium Symbioses.Mol Plant Microbe Interact. 2017 Sep;30(9):739-753. doi: 10.1094/MPMI-04-17-0080-R. Epub 2017 Jul 10. Mol Plant Microbe Interact. 2017. PMID: 28598263
-
The role of endogenous thiamine produced via THIC in root nodule symbiosis in Lotus japonicus.Plant Sci. 2019 Jun;283:311-320. doi: 10.1016/j.plantsci.2019.03.011. Epub 2019 Mar 19. Plant Sci. 2019. PMID: 31128701
-
Hormone modulation of legume-rhizobial symbiosis.J Integr Plant Biol. 2018 Aug;60(8):632-648. doi: 10.1111/jipb.12653. Epub 2018 Jun 5. J Integr Plant Biol. 2018. PMID: 29578639 Review.
-
Primary and Secondary Metabolites in Lotus japonicus.J Agric Food Chem. 2023 Aug 2;71(30):11277-11303. doi: 10.1021/acs.jafc.3c02709. Epub 2023 Jul 19. J Agric Food Chem. 2023. PMID: 37466334 Review.
Cited by
-
Enhanced Disease Susceptibility1 Regulates Immune Response in Lotus japonicus.Int J Mol Sci. 2025 Apr 18;26(8):3848. doi: 10.3390/ijms26083848. Int J Mol Sci. 2025. PMID: 40332572 Free PMC article.
References
-
- Hartmann M., Zeier T., Bernsdorff F., Reichel-Deland V., Kim D., Hohmann M., Scholten N., Schuck S., Bräutigam A., Hölze T., et al. Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell. 2018;173:456.e16–469.e16. doi: 10.1016/j.cell.2018.02.049. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

