IgG from Adult Atopic Dermatitis (AD) Patients Induces Nonatopic Neonatal Thymic Gamma-Delta T Cells (γδT) to Acquire IL-22/IL-17 Secretion Profile with Skin-Homing Properties and Epigenetic Implications Mediated by miRNA
- PMID: 35743310
- PMCID: PMC9224404
- DOI: 10.3390/ijms23126872
IgG from Adult Atopic Dermatitis (AD) Patients Induces Nonatopic Neonatal Thymic Gamma-Delta T Cells (γδT) to Acquire IL-22/IL-17 Secretion Profile with Skin-Homing Properties and Epigenetic Implications Mediated by miRNA
Abstract
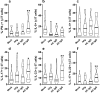
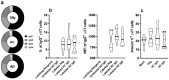
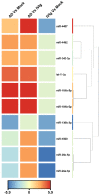
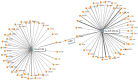
γδT cells mature in the human thymus, and mainly produce IL-17A or IFN-γ, but can also produce IL-22 and modulate a variety of immune responses. Here, we aimed to evaluate whether IgG from AD patients (AD IgG) can functionally modulate thymic nonatopic γδT cells. Thymic tissues were obtained from 12 infants who had not had an atopic history. Thymocytes were cultured in mock condition, or in the presence of either AD IgG or therapeutic intravenous IgG (IVIg). Following these treatments, intracellular cytokine production, phenotype, and microRNA expression profiles were investigated. AD IgG could downregulate α4β7, upregulate CLA, and induce the production of IFN-γ, IL-17, and IL-22 in γδT cells. Although both AD IgG and IVIg could directly interact with γδT cell membranes, AD IgG could reduce γδT cell apoptosis. AD IgG could upregulate nine miRNAs compared to IVIg, and six when compared to the mock condition. In parallel, some miRNAs were downregulated. Target gene prediction and functional analysis indicated that some target genes were enriched in the negative regulation of cellular transcription. This study shows that AD IgG influences the production of IL-17 and IL-22 by intrathymic nonatopic γδT cells, and demonstrates epigenetic implications mediated by miRNAs.
Keywords: IL-17; IL-22; IgG; atopic dermatitis; human; miRNA; p-bodies; thymus; γδT cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

