In Vivo Inhibition of TRPC6 by SH045 Attenuates Renal Fibrosis in a New Zealand Obese (NZO) Mouse Model of Metabolic Syndrome
- PMID: 35743312
- PMCID: PMC9224794
- DOI: 10.3390/ijms23126870
In Vivo Inhibition of TRPC6 by SH045 Attenuates Renal Fibrosis in a New Zealand Obese (NZO) Mouse Model of Metabolic Syndrome
Abstract
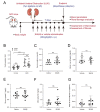
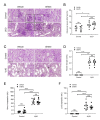
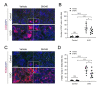
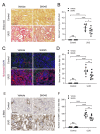
Metabolic syndrome is a significant worldwide public health challenge and is inextricably linked to adverse renal and cardiovascular outcomes. The inhibition of the transient receptor potential cation channel subfamily C member 6 (TRPC6) has been found to ameliorate renal outcomes in the unilateral ureteral obstruction (UUO) of accelerated renal fibrosis. Therefore, the pharmacological inhibition of TPRC6 could be a promising therapeutic intervention in the progressive tubulo-interstitial fibrosis in hypertension and metabolic syndrome. In the present study, we hypothesized that the novel selective TRPC6 inhibitor SH045 (larixyl N-methylcarbamate) ameliorates UUO-accelerated renal fibrosis in a New Zealand obese (NZO) mouse model, which is a polygenic model of metabolic syndrome. The in vivo inhibition of TRPC6 by SH045 markedly decreased the mRNA expression of pro-fibrotic markers (Col1α1, Col3α1, Col4α1, Acta2, Ccn2, Fn1) and chemokines (Cxcl1, Ccl5, Ccr2) in UUO kidneys of NZO mice compared to kidneys of vehicle-treated animals. Renal expressions of intercellular adhesion molecule 1 (ICAM-1) and α-smooth muscle actin (α-SMA) were diminished in SH045- versus vehicle-treated UUO mice. Furthermore, renal inflammatory cell infiltration (F4/80+ and CD4+) and tubulointerstitial fibrosis (Sirius red and fibronectin staining) were ameliorated in SH045-treated NZO mice. We conclude that the pharmacological inhibition of TRPC6 might be a promising antifibrotic therapeutic method to treat progressive tubulo-interstitial fibrosis in hypertension and metabolic syndrome.
Keywords: CKD; NZO mice; SH045; TRPC6; UUO; fibrosis; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Genovese G., Friedman D.J., Ross M.D., Lecordier L., Uzureau P., Freedman B.I., Bowden D.W., Langefeld C.D., Oleksyk T.K., Uscinski Knob A.L., et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- GO766/18-2, GO 766/12-3, SFB 1365, NU 53/12-2, TRR 152/Deutsche Forschungsgemeinschaft
- GO766/18-2, GO 766/12-3, SFB 1365, NU 53/12-2, TRR 152/This work was supported by the Deutsche Forschungsgemeinschaft (DFG) to M.G. (GO766/18-2, GO 766/12-3, SFB 1365), B.N. (NU 53/12-2) and M.S. (TRR 152) and Dr. Werner Jackstädt-Stiftung.
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

