Generation and Characterization of a Tumor Stromal Microenvironment and Analysis of Its Interplay with Breast Cancer Cells: An In Vitro Model to Study Breast Cancer-Associated Fibroblast Inactivation
- PMID: 35743318
- PMCID: PMC9224278
- DOI: 10.3390/ijms23126875
Generation and Characterization of a Tumor Stromal Microenvironment and Analysis of Its Interplay with Breast Cancer Cells: An In Vitro Model to Study Breast Cancer-Associated Fibroblast Inactivation
Abstract
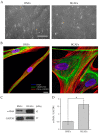
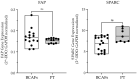
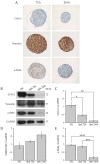
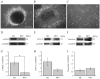
Breast cancer-associated fibroblasts (BCAFs), the most abundant non-cancer stromal cells of the breast tumor microenvironment (TME), dramatically sustain breast cancer (BC) progression by interacting with BC cells. BCAFs, as well as myofibroblasts, display an up regulation of activation and inflammation markers represented by α-smooth muscle actin (α-SMA) and cyclooxygenase 2 (COX-2). BCAF aggregates have been identified in the peripheral blood of metastatic BC patients. We generated an in vitro stromal model consisting of human primary BCAFs grown as monolayers or 3D cell aggregates, namely spheroids and reverted BCAFs, obtained from BCAF spheroids reverted to 2D cell adhesion growth after 216 h of 3D culture. We firstly evaluated the state of activation and inflammation and the mesenchymal status of the BCAF monolayers, BCAF spheroids and reverted BCAFs. Then, we analyzed the MCF-7 cell viability and migration following treatment with conditioned media from the different BCAF cultures. After 216 h of 3D culture, the BCAFs acquired an inactivated phenotype, associated with a significant reduction in α-SMA and COX-2 protein expression. The deactivation of the BCAF spheroids at 216 h was further confirmed by the cytostatic effect exerted by their conditioned medium on MCF-7 cells. Interestingly, the reverted BCAFs also retained a less activated phenotype as indicated by α-SMA protein expression reduction. Furthermore, the reverted BCAFs exhibited a reduced pro-tumor phenotype as indicated by the anti-migratory effect exerted by their conditioned medium on MCF-7 cells. The deactivation of BCAFs without drug treatment is possible and leads to a reduced capability of BCAFs to sustain BC progression in vitro. Consequently, this study could be a starting point to develop new therapeutic strategies targeting BCAFs and their interactions with cancer cells.
Keywords: aggregates; breast cancer; breast cancer cells; breast cancer-associated fibroblasts; conditioned medium; deactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ruocco M.R., Avagliano A., Granato G., Imparato V., Masone S., Masullo M., Nasso R., Montagnani S., Arcucci A. Involvement of Breast Cancer-Associated Fibroblasts in Tumor Development, Therapy Resistance and Evaluation of Potential Therapeutic Strategies. Curr. Med. Chem. 2018;25:3414–3434. doi: 10.2174/0929867325666180309120746. - DOI - PubMed
-
- Avagliano A., Fiume G., Ruocco M.R., Martucci N., Vecchio E., Insabato L., Russo D., Accurso A., Masone S., Montagnani S., et al. Influence of fibroblasts on mammary gland development, breast cancer microenvironment remodeling, and cancer cell dissemination. Cancers. 2020;12:1697. doi: 10.3390/cancers12061697. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

