Applicability of the Thrombin Generation Test to Evaluate the Hemostatic Status of Hemophilia A Patients in Daily Clinical Practice
- PMID: 35743412
- PMCID: PMC9224793
- DOI: 10.3390/jcm11123345
Applicability of the Thrombin Generation Test to Evaluate the Hemostatic Status of Hemophilia A Patients in Daily Clinical Practice
Abstract
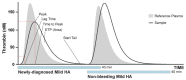
Hemophilia A (HA) is a rare bleeding disorder caused by factor VIII (FVIII) deficiency due to various genetic mutations in the F8 gene. The disease severity inversely correlates with the plasma levels of functional FVIII. The treatment of HA patients is based on FVIII replacement therapy, either following a prophylactic or on-demand regime, depending on the severity of the disease at diagnosis and the patient's clinical manifestations. The hemorrhagic manifestations are widely variable amongst HA patients, who may require monitoring and treatment re-adjustment to minimize bleeding symptoms. Notably, laboratory monitoring of the FVIII activity is difficult due to a lack of sensitivity to various FVIII-related molecules, including non-factor replacement therapies. Hence, patient management is determined mainly based on clinical manifestations and patient-clinician history. Our goal was to validate the ST Genesia® automated thrombin generation analyzer to quantify the relative hemostatic status in HA patients. We recruited a cohort of HA patients from the Principality of Asturias (Spain), following treatment and at a stable non-bleeding phase. The entire cohort (57 patients) had been comprehensively studied at diagnosis, including FVIII and VWF activity assays and F8 genetic screening, and then clinically monitored until the Thrombin Generation Test (TGT) was performed. All patients were recruited prior to treatment administration, at the maximum time-window following the previous dose. Interestingly, the severe/moderate patients had a similar TGT compared to the mild patients, reflecting the non-bleeding phase of our patient cohort, regardless of the initial diagnosis (i.e., the severity of the disease), treatment regime, and FVIII activity measured at the time of the TGT. Thus, TGT parameters, especially the peak height (Peak), may reflect the actual hemostatic status of a patient more accurately compared to FVIII activity assays, which may be compromised by non-factor replacement therapies. Furthermore, our data supports the utilization of combined TGT variables, together with the severity of patient symptoms, along with the F8 mutation type to augment the prognostic capacity of TGT. The results from this observational study suggest that TGT parameters measured with ST Genesia® may represent a suitable tool to monitor the hemostatic status of patients requiring a closer follow-up and a tailored therapeutic adjustment, including other hemophilia subtypes or bleeding disorders.
Keywords: F8; FVIII; Hemophilia A; bleeding; mutation; thrombin generation test.
Conflict of interest statement
This research was funded and promoted by STAGO, which contributed to setting up fundamental questions relevant for the study and reviewing the manuscript. However, the company did not have a role on study design, data collection, analysis, or interpretation. The authors declare no conflict of interest.
Figures

References
-
- White G.C., II, Rosendaal F., Aledort L.M., Lusher J.M., Rothschild C., Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb. Haemost. 2001;85:560. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

