Antibiotic Prescription and In-Hospital Mortality in COVID-19: A Prospective Multicentre Cohort Study
- PMID: 35743662
- PMCID: PMC9224767
- DOI: 10.3390/jpm12060877
Antibiotic Prescription and In-Hospital Mortality in COVID-19: A Prospective Multicentre Cohort Study
Abstract
Background: Since the beginning of the COVID-19 pandemic, empiric antibiotics (ATBs) have been prescribed on a large scale in both in- and outpatients. We aimed to assess the impact of antibiotic treatment on the outcomes of hospitalised patients with moderate and severe coronavirus disease 2019 (COVID-19).
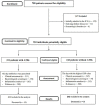
Methods: We conducted a prospective multicentre cohort study in six clinical hospitals, between January 2021 and May 2021.
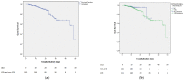
Results: We included 553 hospitalised COVID-19 patients, of whom 58% (311/553) were prescribed antibiotics, while bacteriological tests were performed in 57% (178/311) of them. Death was the outcome in 48 patients-39 from the ATBs group and 9 from the non-ATBs group. The patients who received antibiotics during hospitalisation had a higher mortality (RR = 3.37, CI 95%: 1.7-6.8), and this association was stronger in the subgroup of patients without reasons for antimicrobial treatment (RR = 6.1, CI 95%: 1.9-19.1), while in the subgroup with reasons for antimicrobial therapy the association was not statistically significant (OR = 2.33, CI 95%: 0.76-7.17). After adjusting for the confounders, receiving antibiotics remained associated with a higher mortality only in the subgroup of patients without criteria for antibiotic prescription (OR = 10.3, CI 95%: 2-52).
Conclusions: In our study, antibiotic treatment did not decrease the risk of death in the patients with mild and severe COVID-19, but was associated with a higher risk of death in the subgroup of patients without reasons for it.
Keywords: COVID-19; SARS-CoV-2; antibacterial agents; antibiotics; cohort studies; hospital mortality; mortality; prospective studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Karami Z., Knoop B.T., Dofferhoff A.S.M., Blaauw M.J.T., Janssen N.A., van Apeldoorn M., Kerckhoffs A.P.M., van de Maat J.S., Hoogerwerf J.J., Oever J.T. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: Results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. 2021;53:102–110. doi: 10.1080/23744235.2020.1839672. - DOI - PubMed
-
- COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital|Guidance|NICE. [(accessed on 1 June 2021)]. Available online: https://www.nice.org.uk/guidance/ng173.
-
- Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., Oczkowski S., Levy M.M., Derde L., Dzierba A., et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Crit. Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. - DOI - PMC - PubMed
-
- World Health Organization COVID-19 Clinical Management: Living Guidance. [(accessed on 1 June 2021)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2.
LinkOut - more resources
Full Text Sources
Miscellaneous