Mineralogical and Genomic Constraints on the Origin of Microbial Mn Oxide Formation in Complexed Microbial Community at the Terrestrial Hot Spring
- PMID: 35743847
- PMCID: PMC9224936
- DOI: 10.3390/life12060816
Mineralogical and Genomic Constraints on the Origin of Microbial Mn Oxide Formation in Complexed Microbial Community at the Terrestrial Hot Spring
Abstract
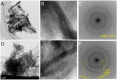
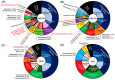
Manganese (Mn) oxides are widespread on the surface environments of the modern Earth. The role of microbial activities in the formation of Mn oxides has been discussed for several decades. However, the mechanisms of microbial Mn oxidation, and its role in complex microbial communities in natural environments, remain uncertain. Here, we report the geochemical, mineralogical, and metagenomic evidence for biogenic Mn oxides, found in Japanese hot spring sinters. The low crystallinity of Mn oxides, and their spatial associations with organic matter, support the biogenic origin of Mn oxides. Specific multicopper oxidases (MCOs), which are considered Mn-oxidizing enzymes, were identified using metagenomic analyses. Nanoscale nuggets of copper sulfides were, also, discovered in the organic matter in Mn-rich sinters. A part of these copper sulfides most likely represents traces of MCOs, and this is the first report of traces of Mn-oxidizing enzyme in geological samples. Metagenomic analyses, surprisingly, indicated a close association of Mn oxides, not only in aerobic but also in anaerobic microbial communities. These new findings offer the unique and unified positions of Mn oxides, with roles that have not been ignored, to sustain anaerobic microbial communities in hot spring environments.
Keywords: anaerobes; biogenic Mn oxides; hot spring; metagenome; multicopper oxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tebo B.M., Bargar J.R., Clement B.G., Dick G.J., Murray K.J., Parker D., Verity R., Webb S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004;32:287–328. doi: 10.1146/annurev.earth.32.101802.120213. - DOI
-
- Akob D.M., Bohu T., Beyer A., Schäffner F., Händel M., Johnson C.A., Merten D., Büchel G., Totsche K.U., Küsel K. Characterization of pH dependent Mn(II) oxidation strategies and formation of a bixbyite-like phase by Mesorhizobium australicum T-G1. Appl. Environ. Microbiol. 2014;80:5086–5097. doi: 10.1128/AEM.01296-14. - DOI - PMC - PubMed
-
- Bohu T., Santelli C.M., Akob D.M., Neu T.R., Ciobota V., Rösch P., Popp J., Nietzsche S., Küsel K. Characterization of pH dependent Mn(II) oxidation strategies and formation of a bixbyite-like Phase by Mesorhizobium Australicum T-G1. Front. Microbiol. 2015;6:734. doi: 10.3389/fmicb.2015.00734. - DOI - PMC - PubMed
-
- Dick G.J., Clement B.G., Webb S.M., Fodrie F.J., Bargar J.R., Tebo B.M. Enzymatic microbial Mn(II) oxidation and Mn biooxide production in the Guaymas Basin deep-sea hydrothermal plume. Geochim. Cosmochim. Acta. 2009;73:6517–6530. doi: 10.1016/j.gca.2009.07.039. - DOI
LinkOut - more resources
Full Text Sources

